Produktinformation
- Bryamycin
- Thiactin
- Alaninamide, <span class="text-smallcaps">L</smallcap>-threonyl-(4S)-2-[(1Z)-1-amino-1-propenyl]-4,5-dihydro-4-thiazolecarbonyl-2-[(1S,2S,3R)-1-amino-2,3-dihydroxy-2-methylbutyl]-4-thiazolecarbonyl-2-[(5R,6S)-6-[2-[(1S,2R)-1-amino-2-hydroxypropyl]-4-thiazolyl]-5-[[N-[(7R,8S)-2-carboxy-7,8-dihydro-8-hydroxy-4-[(1S)-1-hydroxyethyl]-7-quinolinyl]-<smallcap>L</smallcap>-isoleucyl-<smallcap>L</smallcap>-alanyl-2,3-didehydroalanyl-<smallcap>L</span>-alanyl]amino]-5-(4-carboxy-2-thiazolyl)-3,4,5,6-tetrahydro-2-pyridinyl]-4-thiazolecarbonyl-2,3-didehydroalanyl-2,3-didehydro-, (1′→4)-lactone, (4→1)-lactam
- Alaninamide, N-[(7R,8S)-2-carboxy-7,8-dihydro-8-hydroxy-4-[(1S)-1-hydroxyethyl]-7-quinolinyl]-<span class="text-smallcaps">L</smallcap>-isoleucyl-<smallcap>L</smallcap>-alanyl-2,3-didehydroalanyl-<smallcap>L</span>-alanyl-2-[(4aR,11S,14Z,18S,21S,28S,32aS)-4a-amino-21-[(1S,2R)-1,2-dihydroxy-1-methylpropyl]-14-ethylidene-3,4,4a,9,10,11,12,13,14,18,19,20,21,27,28,32a-hexadecahydro-11,28-bis[(1R)-1-hydroxyethyl]-9,12,19,26-tetraoxo-17H,26H-8,5:18,15:25,22:32,29-tetranitrilo-5H,15H-pyrido[3,2-m][1,11,17,24,4,7,20,27]tetrathiatetraazacyclotriacontin-2-yl]-4-thiazolecarbonyl-2,3-didehydroalanyl-2,3-didehydro-, (1→5<sup>28</sup>)-lactone
- Gargon
- N-{3-[(3-amino-3-oxoprop-1-en-2-yl)amino]-3-oxoprop-1-en-2-yl}-2-[(11E)-37-(butan-2-yl)-18-(2,3-dihydroxybutan-2-yl)-11-ethylidene-59-hydroxy-8,31-bis(1-hydroxyethyl)-26,40,46-trimethyl-43-methylidene-6,9,16,23,28,38,41,44,47-nonaoxo-27-oxa-3,13,20,56-tetrathia-7,10,17,24,36,39,42,45,48,52,58,61,62,63,64-pentadecaazanonacyclo[23.23.9.3~29,35~.1~2,5~.1~12,15~.1~19,22~.1~54,57~.0~1,53~.0~32,60~]tetrahexaconta-2(64),4,12(63),19(62),21,29,31,33,51,54,57,60-dodecaen-51-yl]-1,3-thiazole-4-carboxamide (non-preferred name)
- NSC 170365
- NSC 81722
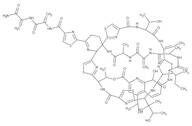
- Thiostrepton
- Thiostrepton A
- Mehr Synonyme anzeigen
- Alaninamide, N-[(7R,8S)-2-carboxy-7,8-dihydro-8-hydroxy-4-[(1S)-1-hydroxyethyl]-7-quinolinyl]-L-isoleucyl-L-alanyl-2,3-didehydroalanyl-L-alanyl-2-[(4aR,11S,14Z,18S,21S,28S,32aS)-4a-amino-21-[(1S,2R)-1,2-dihydroxy-1-methylpropyl]-14-ethylidene-3,4,4a,9,10,11,12,13,14,18,19,20,21,27,28,32a-hexadecahydro-11,28-bis[(1R)-1-hydroxyethyl]-9,12,19,26-tetraoxo-17H,26H-8,5:18,15:25,22:32,29-tetranitrilo-5H,15H-pyrido[3,2-m][1,11,17,24,4,7,20,27]tetrathiatetraazacyclotriacontin-2-yl]-4-thiazolecarbonyl-2,3-didehydroalanyl-2,3-didehydro-, (1→528)-lactone
- Alaninamide, L-threonyl-(4S)-2-[(1Z)-1-amino-1-propenyl]-4,5-dihydro-4-thiazolecarbonyl-2-[(1S,2S,3R)-1-amino-2,3-dihydroxy-2-methylbutyl]-4-thiazolecarbonyl-2-[(5R,6S)-6-[2-[(1S,2R)-1-amino-2-hydroxypropyl]-4-thiazolyl]-5-[[N-[(7R,8S)-2-carboxy-7,8-dihydro-8-hydroxy-4-[(1S)-1-hydroxyethyl]-7-quinolinyl]-L-isoleucyl-L-alanyl-2,3-didehydroalanyl-L-alanyl]amino]-5-(4-carboxy-2-thiazolyl)-3,4,5,6-tetrahydro-2-pyridinyl]-4-thiazolecarbonyl-2,3-didehydroalanyl-2,3-didehydro-, (1′→4)-lactone, (4→1)-lactam
Thiostrepton exhibits antibiotic activity through blockade of ribosome turnover after an elongation step in protein synthesis by inhibiting both the dissociation of elongation factor G (EF-G) from the ribosome and the release of inorganic phosphate from EF-G following GTP hydrolysis. Thiostrepton is also described to show efficacy against cancer cell growth through downregulation of the forkhead box M1 (FOXM1) transcription factor, commonly overexpressed in many malignancies. Thiostrepton is an inhibitor of FOXM1. Thiostrepton has been used as a selectable marker for recombinant Streptomyces cultures. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Chemische Eigenschaften
Technische Anfrage zu: 02-J62332 Thiostrepton, Streptomyces laurentii, 90+%
Wenn Sie ein Angebot anfordern oder eine Bestellung aufgeben möchten, legen Sie stattdessen die gewünschten Produkte in Ihren Warenkorb und fordern Sie dann ein Angebot oder eine Bestellung an aus dem Warenkorb. Es ist schneller, billiger und Sie können von den verfügbaren Rabatten und anderen Vorteilen profitieren.