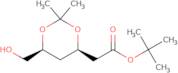
tert-Butyl(3R,5S)-6-hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate
CAS: 124655-09-0
Ref. 3D-FB05616
| 2g | Ausgelaufen | ||
| 5g | Ausgelaufen | ||
| 10g | Ausgelaufen | ||
| 25g | Ausgelaufen | ||
| 50g | Ausgelaufen |
Produktinformation
- 2,4-Dideoxy-3,5-O-(1-methylethylidene)-D-erythro-hexonic acid 1,1-dimethylethyl ester2-[(4R,6S)-6-(Hydroxymethyl)-2,2-dimethyl-1,3- dioxan-4-yl]acetic acid tert-butyl ester
- ((4R,6S)-6-Hydroxymethyl-2,2-dimethyl-[1,3]dioxan-4-yl)acetic acid tert-butyl ester
- (4R-Cis)-6-Hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethyl ester
- (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-aceti acid,1,1-dimethylethyl ester
- (4R-cis)-6-Hydroxymethyl-2, 2-dimethyl-1,3-dioxane-4-aceticacid,1,1-dimethylethyl ester
- (6S-Hydroxymethyl-2,2-dimethyl-[1,3]dioxan-4R-yl)acetic acid tert-butyl ester
- 1,1-Dimethylethyl 2,4-dideoxy-3,5-O-(1-methylethylidene)-<span class="text-smallcaps">D</span>-erythro-hexonate
- 2-[(4R,6S)-6-(Hydroxymethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester
- <span class="text-smallcaps">D</span>-erythro-Hexonic acid, 2,4-dideoxy-3,5-O-(1-methylethylidene)-, 1,1-dimethylethyl ester
- D-6
- Mehr Synonyme anzeigen
- D-erythro-Hexonic acid, 2,4-dideoxy-3,5-O-(1-methylethylidene)-, 1,1-dimethylethyl ester
- Rosuvastatin calcium intermediate C-2
- Tert-Butyl[(4R,6R)-6-Hydroxymethyl-1,3-Dioxan-4-Yl]Acetate
- tert-Butyl 2-[(4R,6S)-2,2-dimethyl-6-(hydroxymethyl)-1,3-dioxan-4-yl]acetate
- tert-Butyl 2-[(4R,6S)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)acetate
- tert-Butyl 2-[(4R,6S)-6-(hydroxymethyl)-2,2-dimethyl[1,3]dioxan-4-yl]acetate
- tert-Butyl(3R,5S)-6-hydroxy-3,5-O-iso-propylidene-3,5-dihydroxyhexanoate
- tert-Butyl(4R-cis)-6-Hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate
- tert-butyl 2,4-dideoxy-3,5-O-(1-methylethylidene)-D-erythro-hexonate
- (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-aceti acid,1,1-dimethylethyl ester(D-6)
- 1,1-Dimethylethyl 2,4-dideoxy-3,5-O-(1-methylethylidene)-D-erythro-hexonate
tert-Butyl(3R,5S)-6-hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate is a chemoenzymatic reductase inhibitor. It is used as an intermediate in the synthesis of other drugs such as lovastatin. The tert-butyl group is chiral and enantioselective. It binds to the active site of HMG CoA reductase and prevents it from catalyzing the conversion of HMG CoA to mevalonic acid. This inhibition leads to decreased production of cholesterol and other lipids.