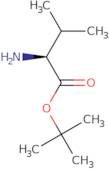
Produktinformation
- (2S)-2-amino-3-methyl-butyric acid tert-butyl ester
- (S)-2-Amino-3-methylbutanoic acid tert-butyl ester
- (S)-Valine tert-butyl ester
- (S)-tert-Butyl 2-amino-3-methylbutanoate
- <span class="text-smallcaps">L</span>-Valine tert-butyl ester
- <span class="text-smallcaps">L</span>-Valine, 1,1-dimethylethyl ester
- H-Val-OBut
- H-Val-OtBu
- L-Valine-Otbu
- L-Valine-T-Butyl Ester
- Mehr Synonyme anzeigen
- Tert-Butyl (2S)-2-Amino-3-Methyl-Butanoate
- Val-OtBu
- Valine tert-butyl ester
- Valine, tert-butyl ester, <span class="text-smallcaps">L</span>-
- tert-Butyl <span class="text-smallcaps">L</span>-valinate
- tert-Butyl L-valinate
- tert-butyl (2S)-2-amino-3-methylbutanoate
- tert-butyl (2S)-2-azanyl-3-methyl-butanoate
- L-Valine, 1,1-dimethylethyl ester
- Valine, tert-butyl ester, L-
- L-Valine tert-butyl ester
Tert-Butyl l-valinate (TBV) is a linker that can be used to synthesize an asymmetric molecule. TBV is typically used in the synthesis of β-unsaturated ketones, which are important for medicinal chemistry and organic synthesis. TBV has been shown to undergo irreversible oxidation with multinuclear and intramolecular hydrogen, as well as nonpolar solvents. TBV has also been shown to react with amino acids under the influence of protonation or acid catalyst. The conjugates formed from TBV have been studied using electrochemical methods.