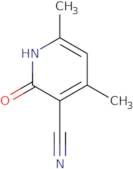
3-Cyano-4,6-dimethyl-2-hydroxypyridine
CAS: 769-28-8
Ref. 3D-FC31164
| 1g | Ausgelaufen | ||
| 2g | Ausgelaufen | ||
| 5g | Ausgelaufen | ||
| 250mg | Ausgelaufen | ||
| 500mg | Ausgelaufen |
Produktinformation
- 2-Hydroxy-4,6-dimethylnicotinonitrile
- (3R)-4,6-dimethyl-2-oxo-2,3-dihydropyridine-3-carbonitrile
- (3S)-4,6-dimethyl-2-oxo-2,3-dihydropyridine-3-carbonitrile
- 1,2-Dihydro-4,6-dimethyl-2-oxo-3-pyridinecarbonitrile
- 2-Hydroxy-4,6-Dimethylnicotinonitrile
- 2-Hydroxy-4,6-Dimethylpyridine-3-Carbonitrile
- 3-Cyano-4,6-dimethyl-2(1H)-pyridinone
- 3-Cyano-4,6-dimethyl-2(1H)-pyridone
- 3-Cyano-4,6-dimethyl-2-pyridone
- 3-Pyridinecarbonitrile, 1,2-dihydro-4,6-dimethyl-2-oxo-
- Mehr Synonyme anzeigen
- 4,6-Dimethyl-2-Oxo-1,2-Dihydropyridine-3-Carbonitrile
- 4,6-Dimethyl-2-hydroxynicotinonitrile
- 5-Cyano-6-hydroxy-2,4-lutidine~4,6-Dimethyl-2-hydroxypyridine-3-carbonitrile
- NSC 52066
- NSC 54161
- Nicotinonitrile, 1,2-dihydro-4,6-dimethyl-2-oxo-
3-Cyano-4,6-dimethyl-2-hydroxypyridine is a chemical compound that can be synthesized by an efficient method. It reacts with phosphorus pentachloride and sodium carbonate to form the corresponding chlorosulfonyloxy derivative in good yield. The reaction proceeds via nucleophilic substitution of the hydroxy group of 3-cyano-4,6-dimethylpyridine by the chloride ion from phosphorus pentachloride. The reaction mechanism is based on a kinetic study using fluorescence probe and allylation methods. The molecule has been shown to be an active analogue of the well known fluorescent probe 2,7-dichlorofluorescin. This probe has been used in vivo analysis and silico analysis to study lipid metabolism and protein kinase activity respectively.