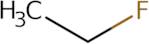
Produktinformation
- Ethane, fluoro-
- Ethyl fluoride
- Ethyl fluoride~FC-161
- F 161
- Hfa 161
- Hfc 161
- Monofluoroethane
- R 161
- R 161 (refrigerant)
Fluoroethane is a chemical compound that is a member of the family of hydrocarbons. It has a boiling point of -34.4 degrees Celsius (-30 degrees Fahrenheit) and is soluble in water to form a colorless solution with a slightly sweet odor. The solubility data indicates that it can be dissolved in water at lower temperatures than other hydrocarbons. Fluoroethane exists as several forms, including hydrogen fluoride (HF), chlorine atom (Cl), and polymer film (PF). In the sample cell, fluorine atoms combine with hydrogen atoms to form HF molecules, which are then converted to HF ions by an electric current. Hydrogen fluoride ions react with chlorine atoms to form ClF molecules, which are then converted back into HF molecules by an electric current. This cycle analysis has been used to determine the activation energies for the reactions of fluorine with hydrogen and chlorine atoms.