Produktinformation
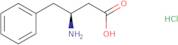
- L-b-HomoPhe-OH·HCl(S)-3-Amino-4-phenylbutyric acid hydrochloride
- (3R)-3-amino-4-phenyl-butanoic acid hydrochloride
- (3S)-3-amino-4-phenylbutanoic acid hydrochloride
- (S)-3-Amino-4-phenylbutanoic acid hydrochloride
- (S)-3-Amino-4-phenylbutyric acid hydrochloride
- (S)-β-Aminobenzenebutanoic acid hydrochloride
- Benzenebutanoic acid, β-amino-, hydrochloride (1:1), (βS)-
- Benzenebutanoic acid, β-amino-, hydrochloride, (S)-
- Benzenebutanoic acid, β-amino-, hydrochloride, (βS)-
- Beta-Homophenylalanine Hydrochloride
- Mehr Synonyme anzeigen
- H-Beta-Homophe-Oh Hcl
- H-Beta-Homophenylalanine Hcl
- H-Beta-Hophe-Oh Hcl
- H-Phe-(C*Ch2)Oh Hcl
- H-β-HoPhe-OH
- H-β-HoPhe-OH.HCl
- L-Beta-Homophenylalanine
- L-Beta-Homophenylalanine Hcl
L-beta-Homophenylalanine hydrochloride is a bifunctional reagent that can be used for the synthesis of amino acids and peptides. It is soluble in water, aqueous solutions, and organic solvents such as chloroform, ethanol, ethers, and hexane. The product has high reactivity at temperatures between 50°C and 100°C. At lower temperatures, it reacts to yield L-homophenylalanine over a period of time. The reaction mechanism starts with the nucleophilic attack by chloride on the electrophilic double bond in bromobenzene to give an intermediate complex. This complex then reacts with hydroxide ion to produce hydrogen chloride gas and benzidine. The remaining steps are similar to those of other reactions involving benzidine. Reaction products include L-homophenylalanine hydrochloride (L-HPA) and hydrogen chloride gas (HCl).
Chemische Eigenschaften
Technische Anfrage zu: 3D-FH49702 L-β-Homophenylalanine hydrochloride
Wenn Sie ein Angebot anfordern oder eine Bestellung aufgeben möchten, legen Sie stattdessen die gewünschten Produkte in Ihren Warenkorb und fordern Sie dann ein Angebot oder eine Bestellung an aus dem Warenkorb. Es ist schneller, billiger und Sie können von den verfügbaren Rabatten und anderen Vorteilen profitieren.