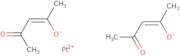
Platinum(II) acetylacetonate
CAS: 15170-57-7
Ref. 3D-FP41664
| 1g | Ausgelaufen | ||
| 2g | Ausgelaufen | ||
| 5g | Ausgelaufen | ||
| 250mg | Ausgelaufen | ||
| 500mg | Ausgelaufen |
Produktinformation
- (SP-4-1)-Bis(2,4-pentanedionato-κO<sup>2</sup>,κO<sup>4</sup>)platinum
- Bis(2,4-pentanedionato)platinum
- Bis(acetylacetonato)platinum
- Bis(acetylacetonato)platinum(II)
- Platinum acetylacetonate
- Platinum bis(acetylacetonate)
- Platinum(2+) acetylacetonate
- Platinum(II) 2,4-pentanedionate
- Platinum, bis(2,4-pentanedionato)-
- Platinum, bis(2,4-pentanedionato-O,O′)-, (SP-4-1)-
- Mehr Synonyme anzeigen
- Platinum, bis(2,4-pentanedionato-κO,κO′)-, (SP-4-1)-
- Platinum, bis(2,4-pentanedionato-κO<sup>2</sup>,κO<sup>4</sup>)-, (SP-4-1)-
- Platinumacetyacetonate
- Pt(acac)<sub>2</sub>
- platinum(2+) bis[(2Z)-4-oxopent-2-en-2-olate]
Platinum(II) acetylacetonate is a chemical compound that is used as a catalyst in organic synthesis. It can be synthesized using the following methods: 1) reaction of an aryl halide with diphenyl ether; 2) preparation from platinum metal and acetic anhydride in chloroform; 3) mixing of an aryl halide and platinum metal powder in water-ethanol or water-toluene. Platinum(II) acetylacetonate has been shown to have high reactivity as a catalyst for reactions such as the Friedel-Crafts alkylation, nitration, and acylation reactions. This catalyst also has been found to be stable at high temperatures and pressures and capable of undergoing hydrolysis when heated with water. Platinum(II) acetylacetonate has also been shown to have strong effects on the physical properties of many molecules, including fluorescence emission, laser ablation rates, FTIR spect