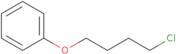
Produktinformation
- (4-Chlorobutoxy)benzene4-Chlorobutyl phenyl ether
- (4-Chlorobutoxy)benzene
- 1-Chloro-4-phenoxybutane
- 4-Chlorobutyl phenyl ether
- 4-Phenoxy-1-chlorobutane
- Benzene, (4-chlorobutoxy)-
- Ether, 4-chlorobutyl phenyl
- NSC 78903
The cleavage of the C-O bond in 4-phenoxybutyl chloride is accomplished by a number of different mechanisms. The most common reaction is the cleavage of the C-O bond from the protonated form to give a cyclic ether. This process is catalyzed by dimethylformamide, which has been shown to be an effective catalyst for this type of reaction. The cleavage can also occur through deuterium exchange between two molecules, followed by hydrogen abstraction and bond cleavage. The electrophilic attack on the C-O bond can also occur through perchlorate ion, followed by proton abstraction and bond cleavage. Finally, there is a mechanism that involves cyclobutane as an intermediate where it undergoes a Diels-Alder reaction with tetramethylammonium and ethylene.