Produktinformation
- (T-4)-Diaqua[ethanedioato(2-)-κO<sup>1</sup>,κO<sup>2</sup>]iron
- Ethanedioic acid, iron(2+) salt (1:1), dihydrate
- Ferrous Oxalate
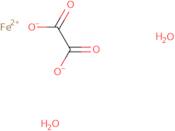
- Ferrous Oxalate Dihydrate
- Ferrous oxalate (FeC<sub>2</sub>O<sub>4</sub>) dihydrate
- Iron oxalate (FeC<sub>2</sub>O<sub>4</sub>) dihydrate
- Iron oxalate hydrate (FeC<sub>2</sub>O<sub>4</sub>.2H<sub>2</sub>O)
- Iron(2+) Ethanedioate Hydrate (1:1:2)
- Iron(2+) oxalate dihydrate
- Iron, diaqua(ethanedioato(2-)-kappao1,kappao2)-, (T-4)-
- Mehr Synonyme anzeigen
- Iron, diaqua[ethanedioato(2-)-O,O′]-
- Iron, diaqua[ethanedioato(2-)-κO<sup>1</sup>,κO<sup>2</sup>]-, (T-4)-
- Ironoxalatedihydrateminyellowpowder
- Oxalic acid, iron(2+) salt (1:1), dihydrate
- Iron (II) oxalate dihydrate
Iron(II)oxalatedi hydrate is a model system that can be used to study the reaction mechanism of the oxidation of hydrocarbons. It is created by forming an iron oxalate salt from iron(III) oxide and oxalic acid. The reaction solution contains a cationic surfactant, which stabilizes the particle in solution. The protonated form of the oxalatedi hydrate has a kinetic rate constant of 1.4 x 10^-5 s^-1 at 25°C and pH 7, with a structural analysis showing that this particle is composed of a lithium ion surrounded by four oxalates and two water molecules. This particle reacts with hydrocarbons to produce carbon dioxide and hydrogen gas, which are then oxidized to form water and carbon monoxide.
Chemische Eigenschaften
Technische Anfrage zu: 3D-GAA04725 Iron(II) oxalate dihydrate
Wenn Sie ein Angebot anfordern oder eine Bestellung aufgeben möchten, legen Sie stattdessen die gewünschten Produkte in Ihren Warenkorb und fordern Sie dann ein Angebot oder eine Bestellung an aus dem Warenkorb. Es ist schneller, billiger und Sie können von den verfügbaren Rabatten und anderen Vorteilen profitieren.