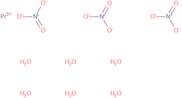
Produktinformation
- Nitric acid, praseodymium(3+) salt, hexahydrate
- Praseodymium Nitrate Hydrate (1:3:6)
- Praseodymium nitrate (Pr(NO<sub>3</sub>)<sub>3</sub>), hexahydrate
- Praseodymium nitrate hexahydrate
- Praseodymium trinitrate hexahydrate
- Praseodymiumnitratetetrahydrate
- Praseodymium nitrate (Pr(NO3)3), hexahydrate
Praseodymium (III) nitrate hexahydrate is a compound that belongs to the group of coordination complexes. It has a particle shape and can be produced by heating praseodymium nitrate in water. Praseodymium (III) nitrate hexahydrate is soluble in water, but insoluble in organic solvents, such as benzene or acetone. The metal-oxygen bonds are broken when the complex reacts with water vapor to form hydroxyl groups. This reaction is reversible and can be used to synthesize praseodymium oxide. The synthesis of this compound is achieved by reacting zirconium oxide with an aqueous solution containing hydrogen peroxide, sodium hydroxide, and ammonium nitrate. Praseodymium (III) nitrate hexahydrate has been shown to have supercritical properties at temperatures above 700 °C and at pressures between 3 and 400 bar. FT-IR spect