Produktinformation
- DICOBALT OCTACARBONYL
- Carbon Monooxide - Cobalt (4:1)
- Carbon Monoxide
- Cobalt
- Cobalt carbonyl
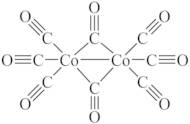
- Cobalt carbonyl (Co2(CO)8)
- Cobalt carbonyl (Co<sub>2</sub>(CO)<sub>8</sub>)
- Cobalt octacarbonyl (Co2(CO)8)
- Cobalt octacarbonyl (Co<sub>2</sub>(CO)<sub>8</sub>)
- Cobalt tetracarbonyl dimer
- Mehr Synonyme anzeigen
- Cobalt, di-μ-carbonylhexacarbonyldi-, (Co-Co)
- Cobaltous
- Di-μ-carbonylhexacarbonyldicobalt
- Dicobalt Octacarbonyl
- Dicobalt carbonyl (Co2(CO)8)
- Dicobalt carbonyl (Co<sub>2</sub>(CO)<sub>8</sub>)
- Kobalt-Tetracarbonyl, Dimer
- Methanone
- Octacarbonildicobalto
ALD Material
Atomic layer deposition (ALD) is a chemically self-limiting deposition technique that is based on the sequential use of a gaseous chemical process. A thin film (as fine as -0.1 Å per cycle) results from repeating the deposition sequence as many times as needed to reach a certain thickness. The major characteristic of the films is the resulting conformality and the controlled deposition manner. Precursor selection is key in ALD processes, namely finding molecules which will have enough reactivity to produce the desired films yet are stable enough to be handled and safely delivered to the reaction chamber.
CVD Material
The growth of thin films via chemical vapor deposition (CVD) is an industrially significant process with a wide array of applications, notably in microelectronic device fabrication. A volatilized precursor (such as a silane, organometallic or metal coordination complex) is passed over a heated substrate. Thermal decomposition of the precursor produces a thin-film deposit, and ideally, the ligands associated with the precursor are cleanly lost to the gas phase as reaction products. Compared to other thin-film production techniques, CVD offers several significant advantages, most notably the potential for effecting selective deposition and lower processing temperatures. Many metal CVD depositions are autocatalytic. Growth of such thin films is characterized by an induction period, which is a consequence of the higher barriers that relate to the activation of the precursor on a non-native substrate. CVD is the preferred deposition method for fabricating optical storage, as it is a well-established method with good scalability, reproducibility, and uniformity. It is also capable of high rates and good composition control.
Cobalt carbonyl; Octacarbonyldicobalt; Dicobalt octacarbonyl; Cobalt tetracarbonyl dimer
Contains 1-4% hexane for stabilityIn combination with SiH4 forms CoSi by CVDCatalyst for conversion of olefins, alkynes, and CO to cyclopentenones