Produktinformation
- (Trimethoxysilyl)benzene
- A 153
- A 153(silane derivative)
- Ay 43-040
- Az 6207
- Benzene, (trimethoxysilyl)-
- Cp0330
- Dc 6124
- Dynasylan 9165
- Feniltrimetoxisilane
- Mehr Synonyme anzeigen
- Geniosil XL 70
- Huls/ Petrarch 04330
- KH 610 (coupling agent)
- Kbm 103
- Kh 610
- Kh 631
- Ls 2570
- Ls 2750
- Nsc 93925
- Ofs 6124
- P 0330
- PhTMO
- PhTMS
- Phenytrimethoxysilane
- Po 330
- Ptms
- Pts 31
- R 00602
- SiSiB PC 8131
- Silane, trimethoxyphenyl-
- Trimethoxyphenylsilan
- Trimethoxyphenylsilane
- Trimethoxysilylbenzene
- Tsl 8173
- X 40-175
- Xiameter OFS 6124
- Z 6071
- Z 6124
- Z 6125
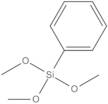
- Phenyltrimethoxysilane
Arylsilane Cross-Coupling Agent
The cross-coupling reaction is a highly useful methodology for the formation of carbon-carbon bonds. It involves two reagents, with one typically being a suitable organometallic reagent - the nucleophile - and the other a suitable organic substrate, normally an unsaturated halide, tosylate or similar - the electrophile.
Aromatic Hydrophobic Trialkoxy Silane
Aliphatic, fluorinated aliphatic or substituted aromatic hydrocarbon substituents are the hydrophobic entities which enable silanes to induce surface hydrophobicity. The organic substitution of the silane must be non-polar. The hydrophobic effect of the organic substitution can be related to the free energy of transfer of hydrocarbon molecules from an aqueous phase to a homogeneous hydrocarbon phase. A successful hydrophobic coating must eliminate or mitigate hydrogen bonding and shield polar surfaces from interaction with water by creating a non-polar interphase. Although silane and silicone derived coatings are in general the most hydrophobic, they maintain a high degree of permeability to water vapor. This allows coatings to breathe and reduce deterioration at the coating interface associated with entrapped water. Since ions are not transported through non-polar silane and silicone coatings, they offer protection to composite structures ranging from pigmented coatings to rebar reinforced concrete. A selection guide for hydrophobic silanes can be found on pages 22-31 of the Hydrophobicity, Hydrophilicity and Silane Surface Modification brochure.
Phenyltrimethoxysilane; Trimethoxysilylbenzene
Viscosity, 25 °C: 2.1 cStVapor pressure, 108 °: 20 mmDipole moment: 1.77Dielectric constant: 4.44Cross-couples w/ aryl bromides w/o fluoride and w/ NaOHHigh yields w/ Pd and carbene ligandsCross-coupled in presence of aryl aldehydeUndergoes 1,4-addition to enones. 1,2- and 1,4-addition to aldehyde undergoes coupling and asymmetric coupling w/ α-bromoestersReacts with 2° amines to give anilinesN-arylates nitrogen heterocyclesCross-coupled w/ alkynyl bromides and iodidesIntermediate for high temperature silicone resinsHydrophobic additive to other silanes with excellent thermal stabilityCross couples with aryl halidesPhenylates heteroaromatic carboxamidesDirectly couples with primary alkyl bromides and iodidesConverts arylselenyl bromides to arylphenylselenidesUsed in nickel-catalyzed direct phenylation of C-H bonds in heteroaromatic systems, benzoxazolesImmobilization reagent for aligned metallic single wall nanotubes (SWNT)Standard grade available, SIP6822.0Extensive review of silicon based cross-coupling agents: Denmark, S. E. et al. "Organic Reactions, Volume 75" Denmark, S. E. ed., John Wiley and Sons, 233, 2011