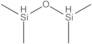
1,1,3,3-TETRAMETHYLDISILOXANE, 98%
CAS: 3277-26-7
Ref. 3H-SIT7546.0
| 14kg | Nachfragen | ||
| 250g | Nachfragen | ||
| 1.5kg | Nachfragen | ||
| 145kg | Nachfragen |
Produktinformation
- 1,1,3,3-Tetramethyl-1,3-dihydrodisiloxane
- 1,1,3,3-Tetramethyl-1,3-disiloxane
- 1,1,3,3-Tetramethyldisiloxan
- 1,1,3,3-Tetramethyldisiloxane
- 1,1,3,3-Tetrametildisiloxano
- 1,3-Dihydrogen-1,1,3,3-tetramethyldisiloxane
- 1,3-Dihydrotetramethyldisiloxane
- Bis(dimethylsilyl) ether
- Bis(dimethylsilyl) oxide
- Dimethylsilyl ether
- Mehr Synonyme anzeigen
- Disiloxane, Tetramethyl-
- Dowsil 3-7010
- Ls 7040
- Nsc 155369
- T 2030
- T 2030 (siloxane)
- Tetramethyldisiloxane
- [(Dimethylsilyl)oxy]dimethylsilane
- sym-Tetramethyldisiloxane
- Disiloxane, 1,1,3,3-tetramethyl-
- 1,1,1,3-tetramethyldisiloxane
- TMDSO
- 1,1,3,3-tetramethyldisiloxane-1,3-diyl
- Tetramethyldiydrodisiloxane
- Hydrogen containing double head 1,1,3,3-Tetramethyldisiloxane
Alkenylsilane Cross-Coupling Agent
The cross-coupling reaction is a highly useful methodology for the formation of carbon-carbon bonds. It involves two reagents, with one typically being a suitable organometallic reagent - the nucleophile - and the other a suitable organic substrate, normally an unsaturated halide, tosylate or similar - the electrophile.
ALD Material
Atomic layer deposition (ALD) is a chemically self-limiting deposition technique that is based on the sequential use of a gaseous chemical process. A thin film (as fine as -0.1 Å per cycle) results from repeating the deposition sequence as many times as needed to reach a certain thickness. The major characteristic of the films is the resulting conformality and the controlled deposition manner. Precursor selection is key in ALD processes, namely finding molecules which will have enough reactivity to produce the desired films yet are stable enough to be handled and safely delivered to the reaction chamber.
Siloxane-Based Silane Reducing Agent
Organosilanes are hydrocarbon-like and possess the ability to serve as both ionic and free-radical reducing agents. These reagents and their reaction by-products are safer and more easily handled and disposed than many other reducing agents. The metallic nature of silicon and its low electronegativity relative to hydrogen lead to polarization of the Si-H bond yielding a hydridic hydrogen and a milder reducing agent compared to aluminum-, boron-, and other metal-based hydrides. A summary of some key silane reductions are presented in Table 1 of the Silicon-Based Reducing Agents brochure.
1,1,3,3-Tetramethyldisiloxane; 1,1-Dihydro-1,1,3,3-tetramethyldisiloxane; TMDO; TMDS
Viscosity, 20 °C: 0.56 cStΔHcomb: 4,383 kJ/molΔHvap: 30.3 kJ/molVapor pressure, 27 °C: 194.8 mmReduces aromatic aldehydes to benzyl halidesEmployed in reductive halogenation of aldehydes and epoxidesUsed to link ferrocenylsilane, polyolefin block copolymers into stable cylindrical formsEndcapper for polymerization of hydride terminated siliconesOrganic reducing agentEmployed in high-yield reduction of amides to amines in the presence of other reducible groupsReduces anisoles to arenesHydrosilylates terminal alkynes to form alkenylsilanes capable of cross-coupling with aryl and vinyl halidesExtensive review of silicon based reducing agents: Larson, G.; Fry, J. L. "Ionic and Organometallic-Catalyzed Organosilane Reductions", Wipf, P., Ed.; Wiley, 2007Extensive review of silicon based cross-coupling agents: Denmark, S. E. et al. "Organic Reactions, Volume 75" Denmark, S. E. ed., John Wiley and Sons, 233, 2011
Chemische Eigenschaften
Technische Anfrage zu: 3H-SIT7546.0 1,1,3,3-TETRAMETHYLDISILOXANE, 98%
Wenn Sie ein Angebot anfordern oder eine Bestellung aufgeben möchten, legen Sie stattdessen die gewünschten Produkte in Ihren Warenkorb und fordern Sie dann ein Angebot oder eine Bestellung an aus dem Warenkorb. Es ist schneller, billiger und Sie können von den verfügbaren Rabatten und anderen Vorteilen profitieren.