Produktinformation
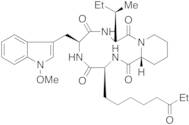
- Cyclo[(2S)-2-amino-8-oxodecanoyl-1-methoxy-L-tryptophyl-L-isoleucyl-(2R)-2-piperidinecarbonyl]
- Cyclo(8-oxo-L-2-aminodecanoyl-1-methoxy-L-tryptophyl-L-isoleucyl-D-2-piperidinecarbonyl)
- Apicidin Ia
- OSI 2040
- Cyclo-L-(2-Amino-8-Oxodeacanoyl)-L-(N-Methoxy-Tryptophan)-L-Isoleucyl-D-Pipecolinyl
- Cyclo-[L-(2-Amino-8-Oxodecanoyl)-L-(N-Methoxytryptophan)-L-Isoleucyl-D-Pipecolinyl
- Cyclo[(2S)-2-Amino-8-Oxodecanoyl-1-Methoxy-L-Tryptophyl-L-Isoleucyl-(2R)-2-Piperidinexcarbonyl]
- Apicidin, Fusarium Species
- (3S,6S,9S,15aR)-6-[(1-methoxy-1H-indol-3-yl)methyl]-9-[(1S)-1-methylpropyl]-3-(6-oxooctyl)octahydro-2H-pyrido[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10(3H,12H)-tetrone
Applications Apicidin is a potent (nM) cell permeable inhibitor of histone deacetylase. Also, Apicidin exhibits antiprotozoal and potential antimalarial properties. Apicidin has antiproliferative activity on HeLa cells accompanied by cell arrest at the G1 phase. Apicidin induces selective changes in the expression of p21 and gelsolin.
References Larsen, T., et al.: J. Microbiol. Methods, 24, 135 (1995), Bode, H., et al.: ChemBioChem., 3, 619 (2002), Nielsen, K., et al.: J. Agric. Food Chem., 53, 8190 (2005),