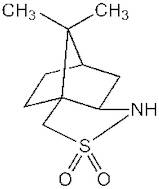
Información del producto
- 10,10-dimethyl-4λ⁶-thia-3-azatricyclo[5.2.1.0²,⁶]decane-4,4-dione
- [3aS-(3aalpha,6alpha,7abeta)]-Hexahydro-8,8-dimethyl-3H-3a,6-methano-2,1-benzisothiazole 2,2-dioxide
- (1S)-(-)-2,10,Camphor sultam
- 8,8-Dimethyloctahydro-4,7-Methano-2,1-Benzothiazole 2,2-Dioxide
- (7aR)-8,8-dimethylhexahydro-3a,6-methano-2,1-benzothiazole 2,2-dioxide
- (3aS,6R,7aR)-8,8-dimethylhexahydro-3a,6-methano-2,1-benzothiazole 2,2-dioxide
- (6R)-8,8-dimethylhexahydro-3a,6-methano-2,1-benzisothiazole 2,2-dioxide
- (3aS,6S,7aS)-8,8-dimethylhexahydro-3a,6-methano-2,1-benzisothiazole 2,2-dioxide
- (-)-Camphorsultam
- (1S)-(-)-2,10-Camphorsultam
- Ver más sinónimos
- (1S)-(-)-2,11-Camphorsultam
(1S,2R)-(-)-10,2-Camphorsultam is used in the asymmetric synthesis of (S)- and (R)-N-Fmoc-S-trityl-α-methylcysteine. It is used as proton source in the synthesis of chiral α,γ-substituted γ-butyrolactones. It is also employed as a chiral probe for the optical resolution by HPLC and X-ray crystallographic determination of the absolute stereochemistry of carboxylic acids. Also used to prepare N-acryloyl derivatives which are employed as dienophiles in asymmetric Diels-Alder reactions. and for other asymmetric transformations. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Propiedades químicas
Consulta técnica sobre: 02-A15897 (1S,2R)-(-)-10,2-Camphorsultam, 99%
Si desea solicitar un presupuesto o realizar un pedido, por favor añada los productos deseados a su carrito y solicite un presupuesto o pedido desde el carrito. Es más rápido, más barato, y podrá beneficiarse de los descuentos y las ventajas disponibles.