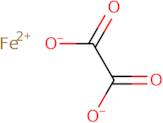
Ferrous oxalate
CAS: 516-03-0
Ref. 3D-AAA51603
| 1kg | Descatalogado | ||
| 50g | Descatalogado | ||
| 100g | Descatalogado | ||
| 250g | Descatalogado | ||
| 500g | Descatalogado |
Información del producto
- Iron, [ethanedioato(2-)-O,O′]-
- Iron, [ethanedioato(2-)-κO1,κO2]-
- Oxalic acid, iron(2+) salt (1:1)
- [Ethanedioato(2-)-κO1,κO2]iron
Ferrous oxalate is a water treatment agent that reacts with the proton of water to form ferric hydroxide and hydrogen gas. The reaction is highly exothermic, which makes it useful in wastewater treatment. Ferrous oxalate can also be used to remove phosphorus pentoxide from wastewater. The kinetic data for this reaction show that the rate of change of oxidation-reduction potentials follows first order kinetics. This means that the rate at which ferrous oxalate oxidizes hydrogen ions is proportional to the concentration of ferrous oxalate. Oxalates are a type of organic acid found in many plants and animals, including humans. Ferrous oxalate has been shown to react with magnesium salt and hydrochloric acid to form an acid complex, which can then be extracted using gravimetric analysis or electrochemical impedance spectroscopy.