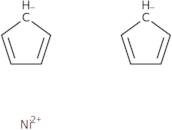
Bis(cyclopentadienyl)nickel(II)
CAS: 1271-28-9
Ref. 3D-BAA27128
| 10g | A consultar | ||
| 25g | A consultar | ||
| 50g | A consultar |
Información del producto
- 1271-28-9
- Bis(Η5-2,4-Ciclopentadien-1-Il)Niquel
- Bis(Η5-2,4-Cyclopentadiene-1-Yl)Nickel
- Bis(η5-cyclopenta-2,4-dien-1-yl)nickel
- Bis(η5-cyclopentadienyl)nickel
- Bis(η<sup>5</sup>-2,4-cyclopentadien-1-yl)nickel
- Bis(η<sup>5</sup>-cyclopentadienyl)nickel
- Cyclopenta-1,3-Diene
- Cyclopentane
- Di-π-Cyclopentadienylnickel
- Ver más sinónimos
- Dicyclopenta-1,3-Dien-1-Ylnickel
- Dicyclopentadienylnickel
- Nickel
- Nickel(2+) Dicyclopenta-2,4-Dienide
- Nickel, bis(η5-2,4-cyclopentadien-1-yl)-
- Nickel, bis(η<sup>5</sup>-2,4-cyclopentadien-1-yl)-
- Nickel, di-π-cyclopentadienyl-
- Nickel, dicyclopentadienyl-
- Nickelocene
Bis(cyclopentadienyl)nickel (II) is a molecule that consists of nickel and cyclopentadienyl rings. It has a redox potential of +1.06 V. This compound is synthesized by reacting hydrochloric acid with an aryl halide and nitrogen gas in the presence of boron nitride. Bis(cyclopentadienyl)nickel (II) has been shown to be chemically stable, which may be due to its ability to resist oxidation in air or water. It also has a relatively high transport property, allowing it to be readily transported through membranes and across cell membranes. The compound's redox potential is high enough for it to function as an electrocatalyst in hydrogen evolution reactions, but not high enough for it to catalyze the hydrogen oxidation reaction.
Propiedades químicas
Consulta técnica sobre: 3D-BAA27128 Bis(cyclopentadienyl)nickel(II)
Si desea solicitar un presupuesto o realizar un pedido, por favor añada los productos deseados a su carrito y solicite un presupuesto o pedido desde el carrito. Es más rápido, más barato, y podrá beneficiarse de los descuentos y las ventajas disponibles.