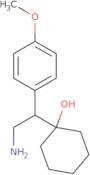
D,L-N,N-Didesmethyl venlafaxine
CAS: 93413-77-5
Ref. 3D-FD21749
| 2mg | Descatalogado | ||
| 5mg | Descatalogado | ||
| 10mg | Descatalogado | ||
| 25mg | Descatalogado | ||
| 50mg | Descatalogado |
Información del producto
- 1-[2-Amino-1-(4-methoxyphenyl)ethyl]cyclohexanol1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanolDinorvenlafaxine
- 1-(4-Methoxyphenyl)-2-Aminoethyl Cyclohexanol Hydrochloride
- 1-[2-Amino-(4-methoxy phenyl)ethyl]cyclohexanol hydrochloride
- 1-[2-Amino-1-(4-Methoxyphenyl)Ethyl]Cyclohexanol
- 1-[2-Amino-1-(4-Methyoxyphenyl)ethyl] Cyclohexanol.HCL
- 1-[2-Amino-1-(4-methoxyphenyl)ethyl]cyclohexan-1-ol
- 1-[2-Amino-1-(4-methoxyphenyl)ethyl]cyclohexanol HCL
- 1-[2-Amino-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride
- 1-[2-Amino-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride (1:1)
- 1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanol
- Ver más sinónimos
- 1-[2-Amino-1-(p-methoxyphenyl)ethyl]cyclohexanol hydrochloride
- 2-(1-Hydroxy-1-cyclohexyl)-2-(4-methoxyphenyl)ethylamine
- 2-(4-Methoxyphenyl)-2-(1-hydroxycyclohexyl)-ethylamine
- Cyclohexanol, 1-[2-amino-1-(4-methoxyphenyl)ethyl]-
- Cyclohexanol,1-[2-amino-1-(4-methoxyphenyl)ethyl]-, hydrochloride
- Cyclohexanol,1-[2-amino-1-(4-methoxyphenyl)ethyl]-, hydrochloride, (?à)-
- Dinorvenlafaxine
- N,N-Didesmethylvenlafaxine
- N,N-Didesmethylvenlafaxine Hydrochloride
- cyclohexanol, 1-[2-amino-1-(4-methoxyphenyl)ethyl]-, hydrochloride (1:1)
D,L-N,N-Didesmethyl venlafaxine is a primary amino acid with the chemical formula C9H11NO2. It is an environmental contaminant that is found in wastewater and has been found to be resistant to biodegradation. D,L-N,N-Didesmethyl venlafaxine can be synthesized from L-cyclohexanol by treating it with hydrochloric acid and converting the resulting diol into an amine. This compound may bioaccumulate in tissues due to its lipophilic nature. The NMR spectrum of this compound displays two signals at δ 4.71 ppm and δ 5.55 ppm corresponding to the protons on the methylene bridge between C2 and C3 and the proton on C3 respectively. Reaction with hydrochloric acid leads to a cyclic imine intermediate that can undergo ring opening with either water or methanol to produce D,L-N