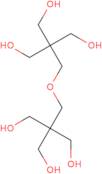
Dipentaerythritol
CAS: 126-58-9
Ref. 3D-FD34700
| 1kg | Descatalogado | ||
| 2kg | Descatalogado | ||
| 5kg | Descatalogado | ||
| 10kg | Descatalogado | ||
| 500g | Descatalogado |
Información del producto
- 1,3-Propanediol, 2,2'-[oxybis(methylene)
- 1,3-Propanediol, 2,2-[Oxybis(Methylene)]bis(2-Hydroxymethyl)
- 2,2'-(Oxydimethanediyl)Bis[2-(Hydroxymethyl)Propane-1,3-Diol]
- 2,2'[Oxybis(Methylen)]Bis[Hydroxymethyl]-1,3-Propandiol
- 2,2,2',2'-Tetrakis(Hidroximetil)-3,3'-Oxidipropan-1-Ol
- 2,2,2',2'-Tetrakis(Hydroxymethyl)-3,3'-Oxydipropan-1-Ol
- 2,2,2',2'-Tetrakis(Hydroxymethyl)-3,3'-Oxydipropane-1-Ol
- 2,2,2,2-Tetrakis(Hydroxymethyl)-3,3-Oxydipropan-1-Ol
- 2,2,6,6,-Tetra(hydroxymethyl)-4-oxaheptane-1,7-diol
- 2,2,6,6-Tetrakis(hydroxymethyl)-4-oxaheptane-1,7-diol
- Ver más sinónimos
- 2,2′-(Oxybis(methylene))bis(2-(hydroxymethyl)propane-1,3-diol)
- 2,2′-[Oxybis(methylene)]bis[2-(hydroxymethyl)-1,3-propanediol]
- 2-([3-Hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl)-2-(hydroxymethyl)-1,3-propanediol
- 2-[[3-Hydroxy-2,2-bis(hydroxymethyl)propoxy]methyl]-2-(hydroxymethyl)propane-1,3-diol
- Bis(pentaerythritol)
- Bis[2,2,2-Tris(Hydroxymethyl)Ethyl] Ether
- Charmor DP 15
- Charmor DP 40
- D 806414
- D-Pe
- D-Pe300
- Di-Penta 93
- Di-Pentarit
- Di-Pentarit 300
- Dipenta 85
- Dipenta 90
- Dipentaerythrit
- Dipentaerythritoltech
- Dipentalide
- Dipentarit SP
- Dipentek
- Dper Ar
- Dper-Hy
- Holtac D
- Lk 48952
- Neulizer N
- Nsc 65881
Dipentaerythritol is a polymeric matrix that is used as a radiation-curable adhesive. It can be prepared by reacting allyl carbonate with phosphotungstic acid in the presence of a film-forming polymer. Dipentaerythritol has reactive hydroxyl groups and forms hydrogen bonds to form long chains. The chemical structures of dipentaerythritol are similar to those of pentaerythritol, which is a substrate for polymerization reactions. This property makes it possible to use dipentaerythritol as a matrix for other materials that are difficult to handle or not compatible with other polymers. The mechanism of reaction involves the formation of an ester bond between two molecules of dihydroxy alcohols, resulting in the formation of an ester group at the end of each molecule.