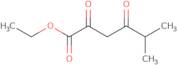
Ethyl5-methyl-2,4-dioxohexanoate
CAS: 64195-85-3
Ref. 3D-FE154269
| 1g | Descatalogado | ||
| 2g | Descatalogado | ||
| 5g | Descatalogado | ||
| 10g | Descatalogado | ||
| 25g | Descatalogado |
Información del producto
- 5-Methyl-2,4-dioxohexanoic acid ethyl ester
- Ethyl 2,4-dioxo-5-methylhexanoate
- Ethyl isobutyrylpyruvate
- Hexanoic acid, 5-methyl-2,4-dioxo-, ethyl ester
- ethyl (2Z)-2-hydroxy-5-methyl-4-oxohex-2-enoate
- Ethyl 5-methyl-2,4-dioxohexanoate
Ethyl 5-methyl-2,4-dioxohexanoate is an intermediate in the preparation of cyclobutene. This compound can be prepared from triphenylphosphine and ethyl acetoacetate via a Wittig reaction. This process is intramolecular, meaning that the two molecules react to form one product without any molecules being lost or added. The Wittig Reaction was first discovered by Georg Wittig in 1953. Intramolecular reactions are often used to synthesize organic compounds with high yields because they do not require any external reagents or catalysts. Ethyl 5-methyl-2,4-dioxohexanoate has also been shown to be reactive with protonation and alkylation reactions.