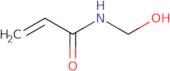
N-Methylolacrylamide
CAS: 924-42-5
Ref. 3D-FM172190
| 5kg | Descatalogado | ||
| 10kg | Descatalogado | ||
| 2500g | Descatalogado |
Información del producto
- Monomethylolacrylamide N-(Hydroxymethyl)-2-propenamid N-(Hydroxymethyl)-acrylamid
- Acrylamide, N-(Hydroxymethyl)-
- Acrylsaeure-(Hydroxymethyl)-Amid
- Cylink NMA
- MH 100 (amide)
- Mh 100
- Monomethylolacrylamide
- N-(Hydroxymethyl)-2-propenamide
- N-(Hydroxymethyl)acrylamid
- N-(Hydroxymethyl)acrylamide
- Ver más sinónimos
- N-(hidroximetil)acrilamida
- N-Mam
- N-Mam P
- N-Methanolacrylamide
- N-Methylol acrylamide
- N-Nbm
- NMA
- Nma 48
- Nma 60
- Nsc 553
- Rocagil BT
- U-Ramin T 80
Dimethyl fumarate (DMF) is a reactive compound that can be synthesized from methyl ethyl acrylate and hydrochloric acid. DMF is used as an intermediate in the production of N-methylolacrylamide (NMA). DMF acts as a monomer in this process, which leads to the formation of NMA. The reaction mechanism for this reaction is not completely understood, but it is believed that the hydrolysis of DMF by water molecules leads to the formation of a carbocation intermediate. This intermediate reacts with methyl ethyl acrylate to form NMA. The carcinogenic potential of DMF has been investigated in animal studies. These studies have shown that rats are more susceptible to carcinogens when exposed to radiation after being given DMF. There have been no studies on the carcinogenic potential of NMA, but there is evidence suggesting that it may be carcinogenic because it contains reactive groups such as nitro groups and carboxy