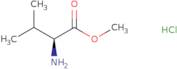
L-Valine methyl ester hydrochloride
CAS: 6306-52-1
Ref. 3D-FV31101
| 10g | Descatalogado | ||
| 25g | Descatalogado | ||
| 50g | Descatalogado | ||
| 100g | Descatalogado |
Información del producto
- (S)-Valine methyl ester hydrochloride
- <span class="text-smallcaps">L</span>-Valine, methyl ester, hydrochloride
- <span class="text-smallcaps">L</span>-Valine, methyl ester, hydrochloride (1:1)
- L-Valine, methyl ester, hydrochloride (1:1)
- Methyl (S)-2-amino-3-methylbutanoate hydrochloride
- Methyl (S)-valinate hydrochloride
- Methyl <span class="text-smallcaps">L</span>-valinate hydrochloride
- Methyl <span class="text-smallcaps">L</span>-valine hydrochloride
- Methyl L-valinate hydrochloride
- Methyl Valinate Hydrochloride
- Ver más sinónimos
- Methylvalinathydrochlorid
- Nsc 197198
- Nsc 22920
- Valinate De Methyle, Chlorhydrate
- Valinato De Metilo, Clorhidrato
- Valine methyl ester hydrochloride
- Valine, methyl ester, hydrochloride, <span class="text-smallcaps">L</span>-
- Valine, methyl ester, hydrochloride, L-
- Val-ome.hcl
- H-Val-OMe.HCl
- L-Valine, methyl ester, hydrochloride
- L-Valine methyl ester HCL
- methyl L-valinate
- (2R)-1-methoxy-3-methyl-1-oxobutan-2-aminium
- L-Valine Methyl Estet HCL
- (S)-Methyl 2-amino-3-methylbutanoate hydrochloride
- H-Val-OMe・HCl
- (2S)-1-methoxy-3-methyl-1-oxobutan-2-aminium
- H-Val-OMe• HCl
L-Valine methyl ester hydrochloride is a chemical compound that is used in analytical chemistry, often as a reagent for the preparation of acid conjugates. It is prepared by the acylation reaction of L-valine and methyl chloride. This process produces an acid chloride, which is then reacted with sodium carbonate to yield L-valine methyl ester hydrochloride. The thermal expansion coefficient of this substance has been shown to be dependent on its concentration and temperature, with values ranging from 6.3 × 10−4/°C at 20 °C to 1.2 × 10−4/°C at 100 °C. The second-order rate constant for the reaction between chlorine and biphenyl has been determined to be 1.7 × 10−5 cm3 molecule−1 s−1 at 25 °C. The elimination rate constant for sodium carbonate in water has been found to be 2 × 10−9 mol2 dm3 molecule