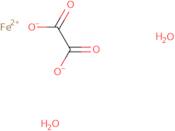
Información del producto
- (T-4)-Diaqua[ethanedioato(2-)-κO<sup>1</sup>,κO<sup>2</sup>]iron
- Ethanedioic acid, iron(2+) salt (1:1), dihydrate
- Ferrous Oxalate
- Ferrous Oxalate Dihydrate
- Ferrous oxalate (FeC<sub>2</sub>O<sub>4</sub>) dihydrate
- Iron oxalate (FeC<sub>2</sub>O<sub>4</sub>) dihydrate
- Iron oxalate hydrate (FeC<sub>2</sub>O<sub>4</sub>.2H<sub>2</sub>O)
- Iron(2+) Ethanedioate Hydrate (1:1:2)
- Iron(2+) oxalate dihydrate
- Iron, diaqua(ethanedioato(2-)-kappao1,kappao2)-, (T-4)-
- Ver más sinónimos
- Iron, diaqua[ethanedioato(2-)-O,O′]-
- Iron, diaqua[ethanedioato(2-)-κO<sup>1</sup>,κO<sup>2</sup>]-, (T-4)-
- Ironoxalatedihydrateminyellowpowder
- Oxalic acid, iron(2+) salt (1:1), dihydrate
- Iron (II) oxalate dihydrate
Iron(II)oxalatedi hydrate is a model system that can be used to study the reaction mechanism of the oxidation of hydrocarbons. It is created by forming an iron oxalate salt from iron(III) oxide and oxalic acid. The reaction solution contains a cationic surfactant, which stabilizes the particle in solution. The protonated form of the oxalatedi hydrate has a kinetic rate constant of 1.4 x 10^-5 s^-1 at 25°C and pH 7, with a structural analysis showing that this particle is composed of a lithium ion surrounded by four oxalates and two water molecules. This particle reacts with hydrocarbons to produce carbon dioxide and hydrogen gas, which are then oxidized to form water and carbon monoxide.
Propiedades químicas
Consulta técnica sobre: 3D-GAA04725 Iron(II) oxalate dihydrate
Si desea solicitar un presupuesto o realizar un pedido, por favor añada los productos deseados a su carrito y solicite un presupuesto o pedido desde el carrito. Es más rápido, más barato, y podrá beneficiarse de los descuentos y las ventajas disponibles.