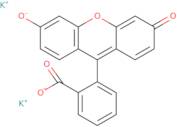
Uranine K
CAS: 6417-85-2
Ref. 3D-GAA41785
| 10g | Descatalogado | ||
| 25g | Descatalogado | ||
| 50g | Descatalogado | ||
| 100g | Descatalogado | ||
| 250g | Descatalogado |
Información del producto
- 12417 Yellow
- D and C Yellow No. 9
- Fluorescein, dipotassium salt
- Fluorescein, potassium deriv., potassium salt
- Japan Yellow 202(2)
- Spiro[isobenzofuran-1(3H),9′-[9H]xanthen]-3-one, 3′,6′-dihydroxy-, dipotassium salt
- Spiro[isobenzofuran-1(3H),9′-[9H]xanthen]-3-one, 3′,6′-dihydroxy-, potassium salt (1:2)
- Uranin K
- Yellow No. 202(2)
- dipotassium 2-(6-oxido-3-oxo-3H-xanthen-9-yl)benzoate
- Ver más sinónimos
- dipotassium 3-oxo-3H-spiro[2-benzofuran-1,9'-xanthene]-3',6'-diolate
Uranine K is a particle that absorbs ultraviolet light. It can be used as a disinfectant for surfaces, such as cavities and teeth, by absorbing uv radiation. Uranine K has low viscosity and is acidic in nature. It has a colorless appearance and is hydroxyl-containing. The chemical structure of Uranine K contains two hydroxyapatite groups, which are the most common inorganic components of bone tissue. Hydroxyapatite is also present in the human body and forms part of the mineralized matrix of bones and teeth. The polymerization initiator in this product is ammonium persulfate, which decomposes into water and sulfur dioxide gas when heated to around 100°C. Ammonium persulfate can be used to initiate polymerization reactions between molecules that contain unsaturated carbon-carbon double bonds (e.g., monomers).