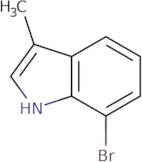
Información del producto
7-Bromo-3-methyl-1H-indole is a heteroaromatic compound that is synthesized in vitro. The synthesis of 7-bromo-3-methyl-1H-indole starts with the reaction of 4,5,6,7 tetrahydrobenzo[c]chromen-2(1H)-one and 2-(trimethylsilyl)ethanol. The indole ring is then formed by the Nef reaction with phosphorous oxychloride in trifluoroacetic acid. This product has been shown to be selective for platelets, and it has also been found to have potent prostanoid activity. The 7 bromo 3 methyl 1 H indole is metabolized by oxidative deamination to yield the oxindoles acetophenone and phenylacetaldehyde. There are three chiral centers in this molecule, which can be made enantiomerically pure using an asymmetric synthesis
Propiedades químicas
Consulta técnica sobre: 3D-LDA91522 7-Bromo-3-methyl-1H-indole
Si desea solicitar un presupuesto o realizar un pedido, por favor añada los productos deseados a su carrito y solicite un presupuesto o pedido desde el carrito. Es más rápido, más barato, y podrá beneficiarse de los descuentos y las ventajas disponibles.