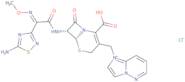
Cefozopran hydrochloride
CAS: 113981-44-5
Ref. 3D-NEA98144
| 5mg | 136,00 € | ||
| 25mg | 213,00 € | ||
| 100mg | 479,00 € | ||
| 250mg | 814,00 € | ||
| 500mg | 1.216,00 € |
Información del producto
- (6R,7R)-7-[2-(5-Amino-1,2,4-thiadiazol-3-yl)-2(Z)-(methoxyimino)acetamido]-3-(imidazo[1,2-b]pyridazinio-1-ylmethyl)-3-cephem-4-carboxylate hydrochloride
- (6R,7R)-7-[[(2E)-2-(5-Amino-1,2,4-thiadiazol-3-yl)-2-methoxyiminoacetyl]amino]-3-(imidazo[2,3-f]pyridazin-4-ium-1-ylmethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid chloride
- 1,2,4-Thiadiazole, imidazo[1,2-b]pyridazinium deriv.
- 1-{[(6R,7R)-7-{[(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(methoxyimino)acetyl]amino}-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl}-1H-imidazo[1,2-b]pyridazin-4-ium chloride
- Cefozopran HCl
- Cefozopran monohydrochloride
- Firstcin
- Imidazo[1,2-b]pyridazinium, 1-[[(6R,7R)-7-[[(2Z)-(5-amino-1,2,4-thiadiazol-3-yl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, chloride
- Imidazo[1,2-b]pyridazinium, 1-[[(6R,7R)-7-[[(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, chloride (1:1)
- Imidazo[1,2-b]pyridazinium, 1-[[7-[[(5-amino-1,2,4-thiadiazol-3-yl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, chloride, [6R-[6α,7β(Z)]]-
- Ver más sinónimos
- SCE-2787.HCl
- [6R-[6alpha,7beta(Z)]]-1-[[7-[[(5-Amino-1,2,4-thiadiazol-3-yl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1H-imidazo[1,2-b]pyridazinium chloride
Cefozopran hydrochloride is a prodrug of cefozopran that is converted to its active form in the body. Cefozopran hydrochloride is used for the treatment of chronic pulmonary infections, specifically those caused by infectious diseases or autoimmune diseases. The drug may also be used for the prevention of bacterial endocarditis in patients who have had a dental procedure. Cefozopran hydrochloride is administered as an injection and has a plasma concentration-time curve that peaks between 1-2 hours after administration. Cefozopran hydrochloride has chemical stability at room temperature, but not at a higher temperature of 37 degrees Celsius. It also has good chemical stability in human serum, which makes it useful for intravenous administration.
Propiedades químicas
Consulta técnica sobre: 3D-NEA98144 Cefozopran hydrochloride
Si desea solicitar un presupuesto o realizar un pedido, por favor añada los productos deseados a su carrito y solicite un presupuesto o pedido desde el carrito. Es más rápido, más barato, y podrá beneficiarse de los descuentos y las ventajas disponibles.