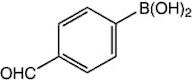
Informations sur le produit
- 4-formylphenylboronic acid
- (4-Formylphenyl)Boronic Acid
- 4-Boronobenzaldehyde
- 4-Formylbenzeneboronic acid
- 4-Formylphenylborate
- B-(4-Formylphenyl)boronic acid
- Benzeneboronic acid, p-formyl-
- Boronic acid, (4-formylphenyl)-
- Boronic acid, B-(4-formylphenyl)-
- p-Formylbenzeneboronic acid
- Voir d'autres synonymes
- p-Formylphenylboronic acid
4-Formylbenzeneboronic acid acts as a reagent used for Palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids, triethylamine-catalyzed three-component Hantzsch condensations, copper-catalyzed nitrations, oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta, palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides, palladium-catalyzed aerobic oxidative cross-coupling reactions. It acts as a reagent used in preparation of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells, a novel protein synthesis inhibitor active against Gram-positive bacteria. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Propriétés chimiques
Question d’ordre technique sur : 02-B25199 4-Formylbenzeneboronic acid, 97%
Si vous souhaitez demander un devis ou passer commande, veuillez plutôt ajouter les produits souhaités à votre panier, puis demander un devis ou passer commande à partir de votre panier. C'est une méthode plus rapide, plus économique, et vous pourrez bénéficier des remises disponibles ainsi que d'autres avantages