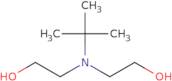
N-tert-Butyldiethanolamine
CAS : 2160-93-2
Ref. 3D-CAA16093
| 1g | Arrêté | ||
| 1l | Arrêté | ||
| 50ml | Arrêté | ||
| 100mg | Arrêté | ||
| 100ml | Arrêté | ||
| 250ml | Arrêté | ||
| 500ml | Arrêté |
Informations sur le produit
- 2,2-(tert-Butylimino)diethanol
- 2,2′-(tert-Butylazanediyl)diethanol
- 2,2′-[(1,1-Dimethylethyl)imino]bis[ethanol]
- 2-[tert-Butyl(2-hydroxyethyl)amino]ethan-1-ol
- 2-[tert-Butyl(2-hydroxyethyl)amino]ethanol
- 3-tert-Butyl-3-aza-1,5-pentanediol
- Amino Alcohol BDEA
- Butyldiethanolamine
- Ethanol, 2,2′-(tert-butylimino)di-
- Ethanol, 2,2′-[(1,1-dimethylethyl)imino]bis-
- Voir d'autres synonymes
- N,N-Bis(2-hydroxyethyl)-tert-butylamine
- N,N-bis(2-hydroxyethyl)-2-methylpropan-2-aminium
- N-tert-Butyl-2,2′-iminodiethanol
- N-tert-Butyl-di(2-hydroxyethyl)amine
- N-tert-Butylbis(2-hydroxyethyl)amine
- N-tert-Butyliminodiethanol
- NSC 525736
- t-BuDEA
- tert-Butylbis(2-hydroxyethyl)amine
- tert-Butyldiethanolamine
N-tert-Butyldiethanolamine is a tertiary amine that has been shown to have genotoxic potential. It can be used as a copper complexing agent to measure the copper concentration in solutions. The titration method involves adding N-tert-Butyldiethanolamine to a solution of copper chloride and piperazine, followed by titration with diethanolamine until the blue color disappears. This process can be used for sample preparation of amines or other compounds with reactive functional groups. N-tert-Butyldiethanolamine has been shown to cause genotoxic activity, but only at high concentrations (>1 mM). This agent is also an organic solvent and may react with other chemicals, such as metals, over time.
The electrochemical studies show that N-tert-Butyldiethanolamine is a good electron donor and will form redox couples with many different types of metal ions,