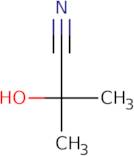
Informations sur le produit
- 2-hydroxyisobutyronitrile
- 2-Cyano-2-hydroxypropane
- 2-Cyano-2-propanol
- 2-Hidroxi-2-Metilpropionitrilo
- 2-Hydroxy-2-Methylpropanenitrile
- 2-Hydroxy-2-cyanopropane
- 2-Hydroxy-2-methylpropionitril
- 2-Hydroxy-2-methylpropionitrile
- 2-Hydroxy-2-methylpropiononitrile
- 2-Hydroxyisobutyronitrile
- Voir d'autres synonymes
- 2-Methyl-2-hydroxypropanenitrile
- 2-Methyl-2-hydroxypropionitrile
- 2-Methyllactonitrile
- 2-Propanone, cyanohydrin
- Acetoncianhidrinei
- Acetoncianidrina
- Acetoncyaanhydrine
- Acetoncyanhydrin
- Acetone cyanhydrin
- Acetonecyanhydrine
- Acetonkyanhydrin
- Ai3-04257
- Brn 0605391
- Ccris 4657
- Cyanhydrine d'acetone
- Cyanhydrine d'acetone [French]
- Dimethyl ketone cyanohydrin
- Einecs 200-909-4
- Hsdb 971
- Lactonitrile, 2-methyl-
- Nsc 131093
- Nsc 7080
- Nsc 977
- Propanenitrile, 2-hydroxy-2-methyl-
- RCRA waste no. P069
- RCRA waste number P069
- Un1541
- Usaf Rh-8
- alpha-Hydroxyisobutyronitrile
- α-Hydroxyisobutyronitrile
Acetone cyanohydrin is a metabolic disorder that has been shown to have toxic effects on the central nervous system. Acetone cyanohydrin is synthesized by reacting acetone with hydrogen cyanide in the presence of an acid catalyst. The synthesis of acetone cyanohydrin proceeds through a nucleophilic addition reaction, followed by elimination and substitution reactions. Acetone cyanohydrin has shown to inhibit locomotor activity in mice, which may be due to its ability to inhibit the conversion of dopamine into norepinephrine and serotonin. Acetone cyanohydrin also inhibits HIV infection, but not as effectively as other drugs such as AZT, ddI, or ddC.