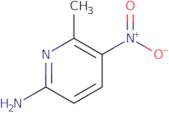
2-Amino-6-methyl-5-nitropyridine
CAS : 22280-62-2
Ref. 3D-FA55142
| 2g | Arrêté | ||
| 5g | Arrêté | ||
| 10g | Arrêté | ||
| 25g | Arrêté | ||
| 50g | Arrêté |
Informations sur le produit
- 2-Amino-5-nitro-6-picoline
- 2-Amino-5-Nitro-6-Methyl Pyridine
- 2-Amino-5-Nitro-6-Methylpyridine
- 2-Amino-5-Nitro-6-Picoline
- 2-Picoline, 6-amino-3-nitro-
- 2-Pyridinamine, 6-methyl-5-nitro-
- 3-Nitro-6-amino-2-picoline
- 6-Amino-2-Methyl-3-Nitropyridine
- 6-Amino-2-Methyl-3-Nitropyridinium
- 6-Amino-3-Nitro-2-Methylpyridine
- Voir d'autres synonymes
- 6-Amino-3-Nitro-2-Picoline
- 6-Methyl-5-Nitro-Pyridin-2-Ylamine
- 6-Methyl-5-Nitropyridin-2-Amine
- 6-Methyl-5-nitro-2-pyridinamine
- 6-Methyl-5-nitropyridin-2-ylamine
- NSC 63855
2-Amino-6-methyl-5-nitropyridine is a structural modification of the parent compound, 6-methylpyridine. It is synthesized by a cross coupling reaction using acetyl chloride and phosphorus pentachloride. It has been shown to have transport properties that are similar to those of pyridine. 2-Amino-6-methyl-5-nitropyridine can be reduced with borohydride or phosphorus oxychloride to produce an aromatic ring. The 2 amino group in 2-amino 6 methyl 5 nitropyridine is deshielded, making it more acidic than its parent compound, 6 methylpyridine. The 2 amino group also causes the molecule to have optical properties similar to those of pyridine. Nitro groups on this molecule make it reactive towards chlorinating agents such as chlorine gas or hypochlorites, which makes it useful for treating tumors in cell lines. In addition, this