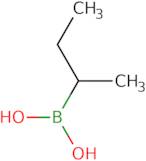
Informations sur le produit
- sec-Butylboronic acid
- (1-Methylpropyl)Boronic Acid
2-Butylboronic acid is a boron-containing organic compound that is used in cross-coupling reactions to form carbon-carbon bonds. It is prepared by the reaction of 2-bromobenzene with sodium diboride in an ether solvent. The yields of this reaction are typically high, giving 99% yield in the case of a Suzuki coupling reaction. Mechanistic studies have shown that the alkylboronic acid catalyzes the coupling by donating a proton to the alkyl halide and then activating it for nucleophilic attack on an electrophile. The anion formed from 2-butylboronic acid can be either a chloride or triflate, depending on whether the electrophile is an organometallic or organocarbon reagent. The regiospecificity of 2-butylboronic acid has been studied using single-crystal x-ray diffraction. The ligands are typically carbox