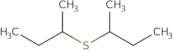
Informations sur le produit
- 2-(Butan-2-Ylsulfanyl)Butane
- 2-Butan-2-ylsulfanylbutane
- 2-[(1-Methylpropyl)thio]butane
- 3,5-Dimethyl-4-thiaheptane
- Butane, 2,2′-thiobis-
- Butane, 2-[(1-methylpropyl)thio]-
- Di(1-methylpropyl) sulfide
- Di-Sec-Butyl Sulphide
- Di-s-Butyl sulfide
- Di-sec-Butyl thioether
- Voir d'autres synonymes
- Di-sec-butyl sulfide
- sec-Butyl sulfide
sec-Butyl sulfide is an organosulfur compound that has a sulfide group attached to a butyl group. The chloride ion can be replaced by other anions or cations, such as nitrate or acetate. This compound is used in the production of dyes, pharmaceuticals, and pesticides. The reaction rate of sec-butyl sulfide is determined by the concentration of the reactants and the temperature. Structural isomers are those compounds that have different structures but the same molecular formula. Sec-butyl sulfide can be either a chiral or a racemic mixture depending on how it reacts with rubrene. The structural theory of organic chemistry states that molecules can have more than one geometric structure that they can adopt at equilibrium because they have more than one way to satisfy valence requirements for each atom in their chemical bonds.