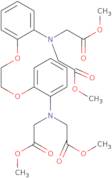
BAPTA-tetramethyl ester
CAS : 125367-34-2
Ref. 3D-FB18138
| 1g | Arrêté | ||
| 2g | Arrêté | ||
| 5g | Arrêté | ||
| 250mg | Arrêté | ||
| 500mg | Arrêté |
Informations sur le produit
- N,N'-[1,2-Ethanediylbis(oxy-2,1-phenylene)]bis[N-(2-methoxy-2-oxoethyl)glycine 1,1'-dimethyl esterTetramethyl 1,2-bis-(2-aminopheno xy)ethane-N,N,N',N'-tetraacetate
- Glycine, N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-(2-methoxy-2-oxoethyl)-, 1,1′-dimethyl ester
- Glycine, N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-(2-methoxy-2-oxoethyl)-, dimethyl ester
- N,N′-[1,2-Ethanediylbis(oxy-2,1-phenylene)]bis[N-(2-methoxy-2-oxoethyl)glycine] 1,1′-dimethyl ester
- Tetramethyl 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetate
BAPTA-tetramethyl ester is a molecule that contains a phosphate group and reactive functional groups. It has a reactive site that can react with other molecules. The phosphate group is used to conjugate BAPTA-tetramethyl ester with other molecules, including fluorescent dyes. The reactive site can also be used to create bifunctional molecules that are able to bind with both the target cell and its environment. BAPTA-tetramethyl ester is most commonly used as a buffer for microparticles in order to keep them from reacting with the environment and giving off light emission. It has been shown that when incubated with target cells, BAPTA-tetramethyl ester will sequester calcium ions, which are required for cellular mechanisms such as protein synthesis and movement of organelles.