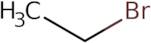
Informations sur le produit
- 2-Bromoethane
- Bromaethan
- Bromethan
- Bromic ether
- Bromoetano
- Ethane, bromo-
- Ethyl bromide
- F 160B1
- Hydrobromic ether
- Monobromoethane
- Voir d'autres synonymes
- Nsc 8824
Bromoethane is a reactive compound that is used as a fluorescent probe for detecting the presence of hemolytic activity in water vapor. It has been shown to depolarize mitochondrial membranes, and it can be used as an antimicrobial agent against bacteria. Bromoethane reacts with sodium carbonate and ethylene diamine to form bromoethane. The reaction mechanism involves the hydroxyl group on bromoethane reacting with hydrogen atoms from ethylene diamine to form water vapor and ethylene glycol. The kinetic energy of the nitrogen atoms in bromoethane causes these reactions to happen quickly. Bromoethane also reacts with carbon disulphide under constant pressure, leading to the formation of water vapor and sulfur dioxide gas.