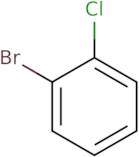
Informations sur le produit
- 1-Bromo-2-chlorobenzene
- 1-Chloro-2-bromobenzene
- 2-Bromo-1-chlorobenzene
- 2-Bromophenyl chloride
- 2-Chlorobromobenzene
- 2-Chlorophenyl bromide
- Ai3-31290
- Benzene, 1-bromo-2-chloro-
- Benzene, bromochloro-
- Bromochlorobenzene
- Voir d'autres synonymes
- Nsc 59694
- o-Bromochlorobenzene
- o-Bromophenyl chloride
- o-Chlorobromobenzene
2-Bromochlorobenzene is a chemical compound that belongs to the group of brominated aromatic compounds. It is used as an intermediate in organic synthesis, often as a precursor to pyrazoles. 2-Bromochlorobenzene can be synthesized by reacting hydrochloric acid with benzene and chloroform in the presence of sodium nitrite. The reactants are heated at temperatures between 50 and 60 °C for 3 hours. This reaction mechanism is shown below:
2-Bromochlorobenzene also reacts with diazonium salt to form an unstable intermediate that decomposes into bromine and chloride ions, which react with each other to form 2-bromochlorobenzene and hydrochloric acid. The reaction solution contains hydrogen chloride gas, which can be removed by bubbling it through water. Chromatography can be used to isolate 2-bromochlorobenzene from the reaction solution by passing