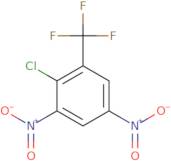
2-Chloro-3,5-dinitrobenzotrifluoride
CAS : 392-95-0
Ref. 3D-FC20056
| 25g | Arrêté | ||
| 50g | Arrêté | ||
| 100g | Arrêté | ||
| 250g | Arrêté | ||
| 500g | Arrêté |
Informations sur le produit
- 2-Chloro-1,5-dinitro-3-(trifluoromethyl)benzene2-Chloro-3,5-dinitro-a,a,a-trifluorotoluene1-Chloro-2,4-dinitro-6-(trifluoromethyl) benzene
- 1,2,3-Trifluoro-4-(Trifluoromethyl)Benzene
- 1,3-Dinitro-5-(Trifluoromethyl)Benzene
- 1-Chloro-2,4-dinitro-6-(trifluoromethyl)benzene
- 2-Chlorine-3,5-Dinitrobenzotrifluoride
- 2-Chloro-1,5-dinitro-3-(trifluoromethyl)-benzene
- 2-Chloro-3,5-Dinitrobenzo Trifluoride
- 2-Chloro-3,5-dinitro-α,α,α-trifluorotoluene
- 2-Chloro-α,α,α-trifluoro-3,5-dinitrotoluene
- Benzene, 2-chloro-1,5-dinitro-3-(trifluoromethyl)-
- Voir d'autres synonymes
- Toluene, 2-chloro-α,α,α-trifluoro-3,5-dinitro-
2-Chloro-3,5-dinitrobenzotrifluoride is a chemical compound that is used as an intermediate in the synthesis of dyes and pharmaceuticals. It reacts with nitrous acid to form 2-chloro-3,5-dinitrobenzoic acid. The hydrogen bond between the hydroxyl group and the chlorine atom in this compound is weak, which leads to a constant value for its spectrometer. This chemical compound has photophysical properties that are dependent on the concentration of solutes. When it is mixed with other solutes such as potassium ion or sulfoxide, it can produce isomers. This chemical compound can be refluxed in a neutral pH environment to form anilines and acetonitrile, two compounds that are important for organic synthesis.