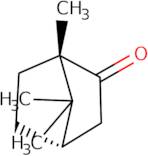
Informations sur le produit
- (-)-Alcanfor
- (-)-Bornan-2-One
- (-)-Camphor
- (1S)-(?-Camphor
- (1S)-1,7,7-Trimethylbicyclo[2.2.1]-2-heptanone
- (1S)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
- (1S,4S)-(-)-Camphor
- (1S,4S)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
- (S)-(-)-Camphor
- 1,7,7-Trimethyl-bicyclo[2.2.1]heptan-2-on = L-Camphor
- Voir d'autres synonymes
- Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1S)-
- Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1S,4S)-
- Camphor, (1S,4S)-(-)-
- NSC 26351
- l-Camphor
Camphor is a colorless, crystalline compound with a strong odor. It is found in the wood of the camphor laurel tree and can be synthetically produced from alpha-pinene. L(-)-camphor has been shown to have antioxidant properties, which may be due to its ability to generate hydrogen peroxide or reactive oxygen species (ROS) at high concentrations. Camphor also has an inhibitory effect on the respiratory chain in mitochondria, which leads to decreased mitochondrial membrane potential. This effect causes physiological effects such as anaesthesia and death when given at high doses. Camphor is used in wastewater treatment systems as an oxidant and disinfectant, with optimum concentrations ranging from 10 ppm to 100 ppm. Camphor also binds to benzalkonium chloride, a common ingredient in household cleaners and disinfectants that inhibits bacterial growth by binding to proteins inside the bacterial cell wall.br>br>
L(-)-Camphor is used in experimental models for studying mitochondrial membrane potentials