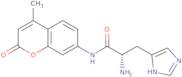
Informations sur le produit
- L-His-AMC
- H-His-AMC
- H-His-AMC.TFA
L-Histidine 7-amido-4-methylcoumarin is a proteolytic enzyme that cleaves proteins at the N-terminal amino acid residue. It was originally purified from a bacterial expression plasmid, but has since been found in other bacteria. The gene product of this enzyme has also been found in bacteriophages and some strains of Proteus mirabilis and Escherichia coli. The biochemical activity of L-Histidine 7-amido-4-methylcoumarin is similar to that of subtilisin, which consists of hydrolyzing peptide bonds with a pK at around 10.5. Spectrometry analysis has shown that L-Histidine 7-amido-4-methylcoumarin is an acidic protein with an optimum pH for activity around 2.2. Hydroxyapatite, polymerase chain reaction (PCR), and cloning experiments have shown that this enzyme may be involved