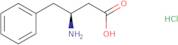
Informations sur le produit
- L-b-HomoPhe-OH·HCl(S)-3-Amino-4-phenylbutyric acid hydrochloride
- (3R)-3-amino-4-phenyl-butanoic acid hydrochloride
- (3S)-3-amino-4-phenylbutanoic acid hydrochloride
- (S)-3-Amino-4-phenylbutanoic acid hydrochloride
- (S)-3-Amino-4-phenylbutyric acid hydrochloride
- (S)-β-Aminobenzenebutanoic acid hydrochloride
- Benzenebutanoic acid, β-amino-, hydrochloride (1:1), (βS)-
- Benzenebutanoic acid, β-amino-, hydrochloride, (S)-
- Benzenebutanoic acid, β-amino-, hydrochloride, (βS)-
- Beta-Homophenylalanine Hydrochloride
- Voir d'autres synonymes
- H-Beta-Homophe-Oh Hcl
- H-Beta-Homophenylalanine Hcl
- H-Beta-Hophe-Oh Hcl
- H-Phe-(C*Ch2)Oh Hcl
- H-β-HoPhe-OH
- H-β-HoPhe-OH.HCl
- L-Beta-Homophenylalanine
- L-Beta-Homophenylalanine Hcl
L-beta-Homophenylalanine hydrochloride is a bifunctional reagent that can be used for the synthesis of amino acids and peptides. It is soluble in water, aqueous solutions, and organic solvents such as chloroform, ethanol, ethers, and hexane. The product has high reactivity at temperatures between 50°C and 100°C. At lower temperatures, it reacts to yield L-homophenylalanine over a period of time. The reaction mechanism starts with the nucleophilic attack by chloride on the electrophilic double bond in bromobenzene to give an intermediate complex. This complex then reacts with hydroxide ion to produce hydrogen chloride gas and benzidine. The remaining steps are similar to those of other reactions involving benzidine. Reaction products include L-homophenylalanine hydrochloride (L-HPA) and hydrogen chloride gas (HCl).
Propriétés chimiques
Question d’ordre technique sur : 3D-FH49702 L-β-Homophenylalanine hydrochloride
Si vous souhaitez demander un devis ou passer commande, veuillez plutôt ajouter les produits souhaités à votre panier, puis demander un devis ou passer commande à partir de votre panier. C'est une méthode plus rapide, plus économique, et vous pourrez bénéficier des remises disponibles ainsi que d'autres avantages