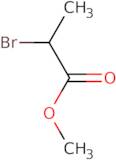
Methyl 2-bromopropanoate
CAS : 5445-17-0
Ref. 3D-FM140187
| 25g | Arrêté | ||
| 50g | Arrêté | ||
| 100g | Arrêté | ||
| 250g | Arrêté | ||
| 500g | Arrêté |
Informations sur le produit
- (.+-.)-2-Bromopropionic acid methyl ester
- (.+-.)-Methyl 2-bromopropionate
- 2-Bromopropanoic acid methyl ester
- 2-Bromopropionate de methyle
- 2-Bromopropionato De Metilo
- <span class="text-smallcaps">DL</span>-α-Bromopropionic acid methyl ester
- DL-α-Bromopropionic acid methyl ester
- Methyl (.+-.)-α-bromopropionate
- Methyl <span class="text-smallcaps">DL</span>-2-bromopropanoate
- Methyl <span class="text-smallcaps">DL</span>-α-bromopropionate
- Voir d'autres synonymes
- Methyl DL-2-bromopropanoate
- Methyl DL-α-bromopropionate
- Methyl α-bromopropanoate
- Methyl-2-brompropionat
- Nsc 21973
- Propionate, 2-Bromo, Methyl
- Propionic acid, 2-bromo-, methyl ester
- methyl (2S)-2-bromopropanoate
- methyl (2R)-2-bromopropanoate
- Propanoic acid, 2-bromo-, methyl ester
Methyl 2-bromopropanoate is a chemical compound that can be synthesized in an asymmetric manner. The reaction of methyl 2-bromopropanoate with hydrochloric acid gives the corresponding carboxylic acid, methyl propanoate, and hydrogen bromide in a 1:1 ratio. It has been shown that methyl 2-bromopropanoate is a potential catalyst for the reduction of chloride to chloride ion via the borohydride reduction method. Methyl 2-bromopropanoate has also been used as a model system for studying halides and copper complexes. Magnetic resonance spectroscopy studies have revealed that this chemical compound has a high redox potential and kinetic properties.