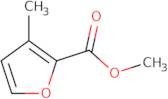
Informations sur le produit
- 3-Methyl-2-furancarboxylic acid methyl ester3-Methyl-2-furoic acid methyl ester3-Methylfuran-2-carboxylic acid methyl ester
- 2-Furancarboxylic acid, 3-methyl-, methyl ester
- 2-Furoic acid, 3-methyl-, methyl ester
- 3-Methyl-2-furoic acid methyl ester
- 3-Methylfuran-2-carboxylic acid methyl ester
- 3-Methylfuran-2-carboxylic acid methyl ester~3-Methyl-2-furoic acid methyl ester~Methyl 3-methylfuran-2-carboxylate
- Methyl 3-Methylfuran-2-Carboxylate
- Methyl 3-methyl-2-furancarboxylate
- Methyl 4-Methylfuran-2-Carboxylate
- NSC 508754
- Voir d'autres synonymes
- Methyl 3-methyl-2-furoate
Methyl 3-methylfuroate is a diketone that is used in organic synthesis. It is synthesized from malonic acid and methanol. The reaction starts with the addition of methanol to malonic acid followed by aldol cyclization, which forms methyl 3-methylfuroate and acetaldehyde as byproducts. This product can be synthesized using fatty acids as well. Methyl 3-methylfuroate can also be synthesized through the activation of chlorinated methyl 3-methylbutanoate with sodium methoxide in methanol, followed by hydrolysis of the resulting ester. The synthesis of methyl 3-methylfuroate from substituted acetates is a more recent development. This reaction requires an isopropyl group, which activates the chlorinated compound, and a tricycle that contains three carbon atoms.