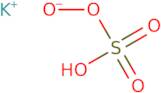
Informations sur le produit
- 10058-23-8
- Caro's acid potassium salt
- Chirox
- Dipotassium Dioxidan-2-Idesulfonate
- Ecocide C
- Monopotassium persulfate
- Peroxymonosulfuric acid, monopotassium salt
- Peroxymonosulfuric acid, potassium salt (1:1)
- Potassium (Hydroperoxysulfonyl)Oxidanide
- Potassium Peroxosulfate
- Voir d'autres synonymes
- Potassium hydrogen dioxidan-2-idesulfonate (1:1:1)
- Potassium hydrogen peroxomonosulfate
- Potassium hydrogen persulfate
- Potassium hydrogenperoxymonosulfate
- Potassium monopersulfate
- Potassium peroxomonosulfate
- Potassium peroxomonosulfate (KHSO<sub>5</sub>)
- Potassium peroxymonosulfate (KHSO<sub>5</sub>)
- Potassium sulfodioxidanide
- Rubysta
- Sulfodioxidanide de potassium
- Potassium peroxymonosulfate (KHSO5)
Potassium peroxymonosulfate (PMS) is a liquid oxidizing disinfectant that is often used in wastewater treatment plants. It has been shown to be effective in the reduction of bacteria and viruses, such as E. coli and H1N1 flu virus, when used in concentrations of 50-100 mg/L. PMS may also be used as an alternative to benzalkonium chloride for the decontamination of surfaces. This chemical can also be used as a chemiluminescent probe to detect nucleic acids, such as DNA and RNA, in a process called polymerase chain reaction (PCR). A model system was developed using sodium citrate and anhydrous sodium peroxide to simulate the chemistry of the reaction between PMS and malonic acid. This system showed that PMS reacted with malonic acid to form hydrogen peroxide, which then reacted with other molecules present to produce chemiluminescence. The untreated group did not show any chem