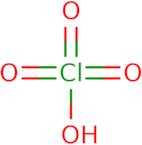
Informations sur le produit
- Acide perchlorique
- Acido Perclorico
- Hydronium perchlorate
- Perchloric acid (H3ClO4)
- Perchloric acid (HClO4)
- Perchloric acid (HClO<sub>4</sub>)
- Perchlorsaeure
- Perchlorsaure
Perchloric acid is a strong mineral acid that is used in various chemical and industrial processes. It has an optimum concentration of 1 M and a ph optimum of 2.5-3.5. Perchloric acid is used for the preparation of samples for analysis by electrochemical methods, such as Langmuir adsorption isotherm, potent antitumor activity, and plasma mass spectrometry. The hydroxyl group on perchloric acid reacts with glycols to produce carboxylic acids or alcohols. This reaction also leads to formation of carbon dioxide gas bubbles and heat, which can be used to oxidize other compounds or to transfer hydrogen ions from one solution to another.
Perchloric acid is also used as a reagent in organic synthesis reactions involving the oxidation of alcohols, phenols, amines, thiols, or sulfides.