Informations sur le produit
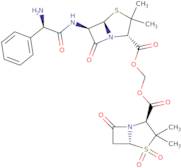
- ({[(2S)-6-{[(2S)-2-amino-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl}oxy)methyl (2S)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide
- ({[(2S,5R,6R)-6-{[(2R)-2-amino-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl}oxy)methyl (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, [[(3,3-dimethyl-4,4-dioxido-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl)carbonyl]oxy]methyl ester, [2S-[2α(2R*,5S*),5α,6β(S*)]]-
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, [[(3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl)carbonyl]oxy]methyl ester, S,S-dioxide, [2S-[2α(2R*,5S*),5α,6β(S*)]]-
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-, [[[(2S,5R)-3,3-dimethyl-4,4-dioxido-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl]oxy]methyl ester, (2S,5R,6R)-
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-aminophenylacetyl]amino]-3,3-dimethyl-7-oxo-, [[[(2S,5R)-3,3-dimethyl-4,4-dioxido-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl]oxy]methyl ester, (2S,5R,6R)-
- Cp 49952
- Sultancillin alkali
- Unacid PD
- Unasyn
- Voir d'autres synonymes
- Vd 1827
- {[(6-{[Amino(Phenyl)Acetyl]Amino}-3,3-Dimethyl-7-Oxo-4-Thia-1-Azabicyclo[3.2.0]Hept-2-Yl)Carbonyl]Oxy}Methyl 3,3-Dimethyl-7-Oxo-4-Thia-1-Azabicyclo[3.2.0]Heptane-2-Carboxylate 4,4-Dioxide
Sultamicillin is a penicillin-type antibiotic that binds to the penicillin-binding protein (PBP) and inhibits bacterial cell wall synthesis. It is used for the treatment of streptococcal pharyngitis, infectious diseases, and other bacterial infections. Sultamicillin has been shown to be effective against a number of bacteria, including Staphylococcus aureus and Streptococcus pyogenes. The drug also has the ability to inhibit multidrug resistance by binding to target enzymes in the cell membrane. This binding prevents bacterial cells from transporting substances across their cell membranes, which can lead to antimicrobial resistance. Structural analysis has revealed that sultamicillin is structurally similar to methicillin with one difference being the dihydrate salt form.