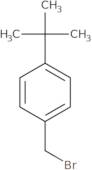
4-tert-Butylbenzyl bromide
CAS : 18880-00-7
Ref. 3D-FT12533
| 1kg | 1.482,00 € | ||
| 2kg | 2.540,00 € | ||
| 100g | 318,00 € | ||
| 250g | 581,00 € | ||
| 500g | 944,00 € |
Informations sur le produit
- 1-Bromomethyl-4-tert-butylbenzeneα-Bromo-4-(tert-butyl)toluene
- 1-(Bromomethyl)-4-(1,1-dimethylethyl)benzene
- 1-(Bromomethyl)-4-Tert-Butylbenzene
- 1-tert-Butyl-4-(bromomethyl)benzene
- 4-(1,1-Dimethylethyl)benzyl bromide
- 4-(Tert-Butyl)Benzyl Bromide
- 4-tert-Butyl-1-bromomethylbenzene
- 4-tert-Butylphenylmethyl bromide
- Benzene, 1-(bromomethyl)-4-(1,1-dimethylethyl)-
- Bromo(4-tert-butylphenyl)methane
- Voir d'autres synonymes
- NSC 186287
- Toluene, α-bromo-p-tert-butyl-
- p-tert-Butylbenzyl bromide
- α-Bromo-p-tert-butyltoluene
Please enquire for more information about 4-tert-Butylbenzyl bromide including the price, delivery time and more detailed product information at the technical inquiry form on this page
Propriétés chimiques
Question d’ordre technique sur : 3D-FT12533 4-tert-Butylbenzyl bromide
Si vous souhaitez demander un devis ou passer commande, veuillez plutôt ajouter les produits souhaités à votre panier, puis demander un devis ou passer commande à partir de votre panier. C'est une méthode plus rapide, plus économique, et vous pourrez bénéficier des remises disponibles ainsi que d'autres avantages