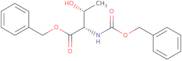
Z-L-Threonine α-benzyl ester
CAS : 16597-50-5
Ref. 3D-FT47315
| 10g | Arrêté | ||
| 25g | Arrêté | ||
| 50g | Arrêté | ||
| 100g | Arrêté | ||
| 250g | Arrêté |
Informations sur le produit
- Z-L-Thr-OBzl
- (2S,3R)-2-[(Benzyloxycarbonyl)amino]-3-hydroxybutanoic acid benzyl ester
- <span class="text-smallcaps">L</span>-Threonine, N-[(phenylmethoxy)carbonyl]-, phenylmethyl ester
- Cbz-L-Threonine Benzyl Ester
- N-(Benzyloxycarbonyl)-<span class="text-smallcaps">L</span>-threonine benzyl ester
- N-(Benzyloxycarbonyl)threonine benzyl ester
- N-[(Phenylmethoxy)carbonyl]-<span class="text-smallcaps">L</span>-threonine phenylmethyl ester
- N-a-CBZ-L-Thr-OBzl
- Threonine, N-carboxy-, dibenzyl ester
- Threonine, N-carboxy-, dibenzyl ester, <span class="text-smallcaps">L</span>-
- Voir d'autres synonymes
- benzyl N-[(benzyloxy)carbonyl]-L-threoninate
- Threonine, N-carboxy-, dibenzyl ester, L-
- N-[(Phenylmethoxy)carbonyl]-L-threonine phenylmethyl ester
- L-Threonine, N-[(phenylmethoxy)carbonyl]-, phenylmethyl ester
The synthesis of Z-L-Threonine α-benzyl ester is accomplished by a two step sequence. The first step is the catalytic conversion of benzyl alcohol to benzyl chloride with sulfuric acid and zinc dust in trifluoromethane. The second step is the reaction of the benzyl chloride with threonine in the presence of tetrazole, followed by purification. The yield obtained was approximately 35%. This method is efficient because it does not require an expensive reagent, such as phosphorus pentoxide, for protection against side reactions. It also does not require high temperatures or vacuum conditions during synthesis.
The synthesized product has carbonyl groups, which can be analyzed using various analytical methods. The synthetic Z-L-threonine α-benzyl ester contains a residue that can be analyzed using various techniques including acid analysis or gas chromatography.
Z-L-Threonine α-benzyl es