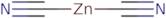Informations sur le produit
- Ai3-28752
- Brn 4124366
- Cyanure de zinc
- Cyanure de zinc [French]
- Dicyanozinc
- Hsdb 1051
- RCRA waste no. P121
- RCRA waste number P121
- Un1713
- Zinc cyanide (Zn(CN)2)
- Voir d'autres synonymes
- Zinc cyanide (Zn(CN)<sub>2</sub>)
- Zinc cyanide [UN1713] [Poison]
- Zinc dicyanide
Zinc cyanide is a chemical compound that is created by the reaction of sodium citrate, hydrochloric acid, and sodium carbonate. It can be used as an adsorbent for the removal of copper from wastewater. When zinc cyanide comes into contact with copper chloride, it forms a complex that has a higher affinity for copper than zinc. This process is reversible and the adsorption capacity can be regenerated by heating the adsorbent material at 110 to 140 degrees Celsius. Zinc cyanide also reacts with anhydrous sodium in the presence of heat to form sodium cyanide, which is then reacted with water under acidic conditions to produce hydrogen cyanide gas.
Methane is produced during this process as well. The metabolic rate for zinc cyanide depends on factors such as temperature and pressure, so there are no hard-and-fast values for this data point.
Propriétés chimiques
Question d’ordre technique sur : 3D-FZ31046 Zinc cyanide
Si vous souhaitez demander un devis ou passer commande, veuillez plutôt ajouter les produits souhaités à votre panier, puis demander un devis ou passer commande à partir de votre panier. C'est une méthode plus rapide, plus économique, et vous pourrez bénéficier des remises disponibles ainsi que d'autres avantages