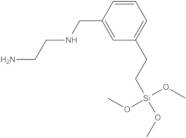
(AMINOETHYLAMINOMETHYL)PHENETHYLTRIMETHOXYSILANE, tech
CAS : 74113-77-2
Ref. 3H-SIA0588.0
| 25g | Arrêté | ||
| 2kg | Arrêté | ||
| 100g | Arrêté |
Informations sur le produit
- 1,2-Ethanediamine, N-[[[2-(trimethoxysilyl)ethyl]phenyl]methyl]-
- 1,2-Ethanediamine, N<sup>1</sup>-[[[2-(trimethoxysilyl)ethyl]phenyl]methyl]-
- Aemp 3
- Aminoethylaminomethylphenethyltrimethoxysilane
- Bis(2,2,6,6-Tetramethylpiperidin-4-Yl) 1,5-Dioxospiro[5.5]Undeca-7,9-Diene-3,3-Dicarboxylate
- N-{4-[2-(trimethoxysilyl)ethyl]benzyl}ethane-1,2-diamine
- N<sup>1</sup>-[[[2-(Trimethoxysilyl)ethyl]phenyl]methyl]-1,2-ethanediamine
- 1,2-Ethanediamine, N1-[[[2-(trimethoxysilyl)ethyl]phenyl]methyl]-
- N1-[[[2-(Trimethoxysilyl)ethyl]phenyl]methyl]-1,2-ethanediamine
Diamino Functional Trialkoxy Silane
Silane coupling agents have the ability to form a durable bond between organic and inorganic materials to generate desired heterogeneous environments or to incorporate the bulk properties of different phases into a uniform composite structure. The general formula has two classes of functionality. The hydrolyzable group forms stable condensation products with siliceous surfaces and other oxides such as those of aluminum, zirconium, tin, titanium, and nickel. The organofunctional group alters the wetting or adhesion characteristics of the substrate, utilizes the substrate to catalyze chemical transformations at the heterogeneous interface, orders the interfacial region, or modifies its partition characteristics, and significantly effects the covalent bond between organic and inorganic materials.
(Aminoethylaminomethyl)phenethyltrimethoxysilane, [N-(2-Aminoethyl)aminomethylphenyl]ethyltrimethoxysilane, N-1-[[[2-(Trimethoxysilyl)ethyl]phenyl]methyl-1-2-ethanediamine
Mixed m-, p- isomersPrimary amine and an internal secondary amine coupling agent for polyimidePhotochemically sensitive (194 nm)Forms self assembled monolayersReagent for charge heterogeneity in micropatterningUsed in microparticle surface modificationDetermined by TGA a 25% weight loss of dried hydrolysates at 435 °CComponent in molecular imprinting of enzymes, see Markowitz, M., et al, Langmuir, 2000, 16, 1759