Informations sur le produit
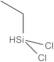
- Dichloro(Ethyl)Silyl
- Dichloroethyl-Silan
- Dichloroethylsilane
- Dichlorosilane
- Monoethyldichlorosilane
- Silane, dichloroethyl-
Tri-substituted Silane Reducing Agent
Organosilanes are hydrocarbon-like and possess the ability to serve as both ionic and free-radical reducing agents. These reagents and their reaction by-products are safer and more easily handled and disposed than many other reducing agents. The metallic nature of silicon and its low electronegativity relative to hydrogen lead to polarization of the Si-H bond yielding a hydridic hydrogen and a milder reducing agent compared to aluminum-, boron-, and other metal-based hydrides. A summary of some key silane reductions are presented in Table 1 of the Silicon-Based Reducing Agents brochure.
Ethyldichlorosilane; Dichloroethylsilane
Viscosity: 0.53 cStΔHform: -391 kJ/molΔHvap: 7.5 kJ/molDipole moment: 2.04 debyeSurface tension: 21.7 mN/mCritical temperature: 252 °CVapor pressure, 21 °C: 100 mmWill form high-boiling polymeric by-products with aqueous work-upExtensive review of silicon based reducing agents: Larson, G.; Fry, J. L. "Ionic and Organometallic-Catalyzed Organosilane Reductions", Wipf, P., Ed.; Wiley, 2007