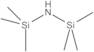
1,1,1,3,3,3-HEXAMETHYLDISILAZANE, 99%
CAS : 999-97-3
Ref. 3H-SIH6110.1
| 25g | À demander | ||
| 14kg | À demander | ||
| 1.5kg | À demander | ||
| 150kg | À demander |
Informations sur le produit
- HMDS, HMDZ
- 1,1,1,3,3,3-Hexamethyldisilazan
- 1,1,1,3,3,3-Hexamethyldisilazane
- 1,1,1,3,3,3-Hexametildisilazano
- 1,1,1-Trimethyl-N-(trimethylsilyl)silanamine
- 12058-1A
- A 166
- A 166 (silazane)
- Bis(trimethylsilyl)amine
- Di(trimethylsilyl)amine
- Voir d'autres synonymes
- Disilazane, 1,1,1,3,3,3-hexamethyl-
- Dn-L 69
- Dow Corning 4-2839
- Dynasylan HMDS
- H 0089
- HMDS (silazane)
- Hexamethyldisilazane
- Hexamethyldisilylamine
- Hexamethylsilazane
- Hmd 3
- Hmds
- Hmds 1
- Hmds 3
- Ls 7150
- Nsc 93895
- OAP
- SE 31 (silazane)
- Se 31
- Sih 6110.1
- Silazan HMN
- Sz 31
- Sz 6079
- Tsl 8802
- Tsr 8802
- Z 6079
- [Dimethyl-(trimethylsilylamino)silyl]methane
- Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-
- 1,1,1,3,3,3,-HEXAMETHYLDISILA-
Alkyl Silane - Conventional Surface Bonding
Aliphatic, fluorinated aliphatic or substituted aromatic hydrocarbon substituents are the hydrophobic entities which enable silanes to induce surface hydrophobicity. The organic substitution of the silane must be non-polar. The hydrophobic effect of the organic substitution can be related to the free energy of transfer of hydrocarbon molecules from an aqueous phase to a homogeneous hydrocarbon phase. A successful hydrophobic coating must eliminate or mitigate hydrogen bonding and shield polar surfaces from interaction with water by creating a non-polar interphase. Although silane and silicone derived coatings are in general the most hydrophobic, they maintain a high degree of permeability to water vapor. This allows coatings to breathe and reduce deterioration at the coating interface associated with entrapped water. Since ions are not transported through non-polar silane and silicone coatings, they offer protection to composite structures ranging from pigmented coatings to rebar reinforced concrete. A selection guide for hydrophobic silanes can be found on pages 22-31 of the Hydrophobicity, Hydrophilicity and Silane Surface Modification brochure.
Silane Cross-Coupling Agent
The cross-coupling reaction is a highly useful methodology for the formation of carbon-carbon bonds. It involves two reagents, with one typically being a suitable organometallic reagent - the nucleophile - and the other a suitable organic substrate, normally an unsaturated halide, tosylate or similar - the electrophile.
Trimethylsilyl Blocking Agent
Used as a protecting group for reactive hydrogens in alcohols, amines, thiols, and carboxylic acids. Organosilanes are hydrogen-like, can be introduced in high yield, and can be removed under selective conditions. They are stable over a wide range of reaction conditions and can be removed in the presence of other functional groups, including other protecting groups. The tolerance of silylated alcohols to chemical transformations summary is presented in Table 1 of the Silicon-Based Blocking Agents brochure.
ALD Material
Atomic layer deposition (ALD) is a chemically self-limiting deposition technique that is based on the sequential use of a gaseous chemical process. A thin film (as fine as -0.1 Å per cycle) results from repeating the deposition sequence as many times as needed to reach a certain thickness. The major characteristic of the films is the resulting conformality and the controlled deposition manner. Precursor selection is key in ALD processes, namely finding molecules which will have enough reactivity to produce the desired films yet are stable enough to be handled and safely delivered to the reaction chamber.
1,1,1,3,3,3-Hexamethyldisilazane; HMDS; HMDZ; Bis(trimethylsilyl)amine
<5 ppm chlorideStandard grade available, SIH6110.0Viscosity: 0.90 cStΔHcomb: 25,332 kJ/molΔHvap: 34.7 kJ/molDipole moment: 0.37 debyeSurface tension: 18.2 mN/mSpecific wetting surface: 485 m2/gVapor pressure, 50 °: 50 mmpKa: 7.55Photoresist adhesion promoterDielectric constant: 1000 Hz: 2.27Ea, reaction w/SiO2 surface: 73.7 kJ/molVersatile silylation reagentCreates hydrophobic surfacesConverts acid chlorides and alcohols to amines in a three-component reactionReacts with formamide and ketones to form pyrimidinesLithium reagent reacts w/ aryl chlorides or bromides to provide primary anilinesUsed to convert ketones to α-aminophosphonates
Propriétés chimiques
Question d’ordre technique sur : 3H-SIH6110.1 1,1,1,3,3,3-HEXAMETHYLDISILAZANE, 99%
Si vous souhaitez demander un devis ou passer commande, veuillez plutôt ajouter les produits souhaités à votre panier, puis demander un devis ou passer commande à partir de votre panier. C'est une méthode plus rapide, plus économique, et vous pourrez bénéficier des remises disponibles ainsi que d'autres avantages